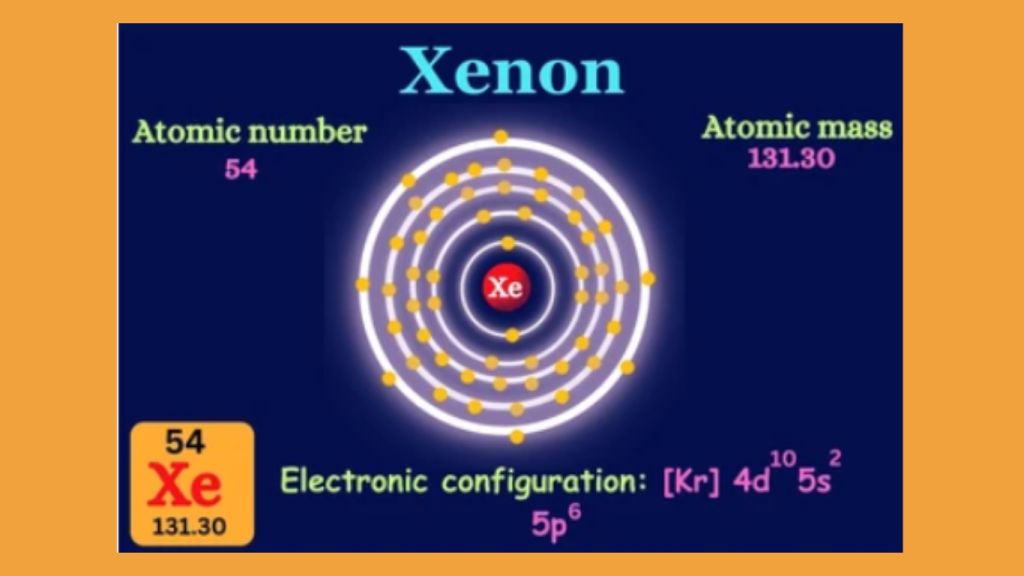
The Xenon electron configuration is [Kr]5s^24d^105p^6. Xenon is an element with a unique electron configuration.
Xenon, a chemical element with the symbol Xe and atomic number 54, is known for its interesting electron configuration. The electron configuration of an atom refers to the distribution of its electrons among its various orbitals. In the case of xenon, its electron configuration can be represented as [Kr]5s^24d^105p^6.
This configuration indicates that xenon has 54 electrons occupying different energy levels and orbitals. Understanding an element’s electron configuration is vital in determining its chemical behavior and reactivity. In the case of xenon, its unique configuration plays a role in its stable nature and its applications in various fields, such as lighting and laser technology.

The Basics Of Electron Configuration
In the realm of chemistry, understanding the electron configuration of an atom is a fundamental concept. This aspect plays a pivotal role in determining the chemical properties and behavior of elements. Essentially, electron configuration refers to the arrangement of electrons within an atom. Delving into the basics of electron configuration unveils fascinating insights into the structure and reactivity of elements.
What Is Electron Configuration?
Electron configuration is the arrangement of electrons within the shells and subshells of an atom. This arrangement follows a set of rules that dictate the distribution of electrons in the orbitals. Each electron occupies a specific energy level, denoted by quantum numbers and subshells, leading to a unique electron configuration for each element.
Importance Of Electron Configuration In Chemistry
The field of chemistry heavily relies on the concept of electron configuration to comprehend the behavior of elements and their interactions in compounds. Predicting the chemical reactivity, bonding capabilities, and physical properties of elements hinges on their electron configurations. It is a foundational tool for understanding the periodic trends and characteristics of the elements, enabling scientists to make informed decisions in chemical processes and applications.
Xenon’s Atomic Structure
Xenon, a noble gas with the atomic number 54, possesses an intriguing atomic structure that sets it apart from other elements on the periodic table. Understanding the arrangement of electrons within xenon is crucial in comprehending its chemical properties and behavior. In this section, we will delve into the number of electrons in xenon and explore how they are arranged within its atomic structure.
Number Of Electrons In Xenon
Xenon contains a total of 54 electrons distributed among its energy levels and subshells. Being the 54th element, xenon is positioned just beneath the noble gases, krypton, and argon, on the periodic table. The number of electrons within an atom corresponds to its atomic number, which in xenon’s case, is 54. This means that xenon has a balanced distribution of protons and electrons, making it electrically neutral.
Arrangement Of Electrons In Xenon
The arrangement of electrons in xenon follows a specific pattern based on the Aufbau principle and the filling order of subshells. The Aufbau principle states that electrons occupy the lowest energy level available before filling higher energy levels. This principle guides the arrangement or electron configuration of xenon, ensuring the stability of the atom.
Let’s break down the electron configuration of xenon:
- The first energy level, which is closest to the nucleus, can hold a maximum of 2 electrons. Xenon’s first energy level is already filled with two electrons.
- The second energy level can hold a maximum of 8 electrons. Xenon’s second energy level is also completely filled with 8 electrons.
- The third energy level, the last and outermost energy level for xenon, has a capacity of 18 electrons. This level holds the remaining 8 electrons in the xenon’s atomic structure.
In summary, xenon’s electron configuration can be represented as follows: 2, 8, 18, 18, 8. These numbers indicate the electrons present in each energy level from innermost to outermost, respectively. This filled arrangement of electrons contributes to xenon’s stability and inertness, making it a valuable element in various applications and industries.
Understanding The Aufbau Principle
The Aufbau principle governs the electron configuration of Xenon, following a pattern of filling orbitals based on increasing energy levels. Understanding this principle helps predict electron arrangement in atoms, which is essential in chemistry and materials science applications.
Understanding the Aufbau Principle
The Aufbau Principle is a fundamental concept in chemistry that helps us understand how electrons are distributed within an atom. In simple terms, it states that electrons fill the lowest energy levels first before moving to higher levels. This principle explains the electron configuration of elements, including xenon. Let’s explore the explanation and application of the Aufbau Principle to understand xenon’s electron configuration better.
Explanation of the Aufbau Principle
The Aufbau Principle states that electrons occupy the available energy levels in a predictable pattern. Starting from the lowest energy level, known as the ground state, electrons fill each orbital before moving to the next one. This principle follows the order of increasing energy, known as the Aufbau sequence. The sequence begins with the filling of the 1s orbital, followed by the 2s, 2p, 3s, and so on.
Application of the Aufbau Principle to Xenon
Xenon, an element in the periodic table, follows the principles of Aufbau to determine its electron configuration. The atomic number of xenon is 54, meaning it has 54 electrons. By applying the Aufbau Principle, we can determine how these electrons are arranged within the atom.
Let’s break down the electron configuration of xenon using the Aufbau Principle:
1. 1s² 2s² 2p⁶: These orbitals comprise the first three energy levels, accommodating 10 electrons.
2. 3s² 3p⁶: These orbitals correspond to the fourth energy level and accommodate an additional 8 electrons.
3. 4s² 3d¹⁰ 4p⁶: The fifth energy level includes the 4s and 4p orbitals, hosting a total of 18 electrons.
4. 5s² 4d¹⁰ 5p⁶: The sixth energy level consists of the 5s and 5p orbitals, accommodating another 18 electrons.
At this point, the arrangements of the first six energy levels account for 54 electrons, equivalent to xenon’s atomic number. The Aufbau Principle helps us understand how these electrons fill the orbitals, providing insight into xenon’s electron configuration.
In summary, the Aufbau Principle outlines how electrons occupy energy levels within an atom, with xenon serving as an example. Applying this principle to xenon helps us determine its electron configuration, which is crucial in understanding this element’s chemical properties and behavior.
Xenon’s Noble Gas Configuration
With a focus on understanding Xenon’s noble gas configuration, it’s crucial to delve into the explanation and determination of this arrangement. Xenon, a noble gas, showcases an exceptional electron configuration contributing to its stability and inert nature.
Explanation Of Noble Gas Configuration
Noble gas configuration refers to the arrangement of electrons in an atom following the pattern of noble gases, which are characterized by their stable electron configurations. Elements strive to attain such configurations to achieve stability, with xenon being no exception.
Determination Of Xenon’s Noble Gas Configuration
Understanding xenon’s noble gas configuration involves examining its atomic structure and the distribution of its electrons in specific energy levels. The configuration can be determined by exploring the periodic table and utilizing the concept of noble gas notation.
Electron Configuration Notation For Xenon
Xenon’s electron configuration notation reveals its unique arrangement of electrons in its atomic structure. Understanding this notation allows for a deeper comprehension of xenon’s chemical properties and its role in various applications.
Introduction
Xenon is a chemical element known for its unique electron configuration. Understanding the electron configuration notation for xenon is essential to comprehend its chemical behavior and properties. In this section, we will explain the electron configuration notation for xenon, specifically focusing on correctly writing it. Let’s dive in!
Explanation Of Electron Configuration Notation
Electron configuration notation is a way to represent the arrangement of electrons within an atom’s electron shells and subshells. The notation uses specific symbols and numbers to indicate the distribution of electrons. This information is vital for understanding an element’s reactivity, bonding abilities, and chemical reactions.
The electron configuration of an atom consists of a series of numbers and letters, with each element having its unique arrangement. The main components of electron configuration notation include the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (m), and the spin quantum number (s).
Writing Xenon’s Electron Configuration
Xenon, denoted by the chemical symbol Xe, has an atomic number of 54. Its electron configuration is represented as [Kr] 5s2 4d10 5p6. Let’s break it down further:
- The symbol [Kr] refers to the electron configuration of the noble gas krypton (Kr), which precedes xenon in the periodic table. The noble gas configuration includes all of the electrons in the previous noble gas, simplifying the notation.
- The 5s orbital can accommodate up to 2 electrons. In xenon’s case, it contains 2 electrons.
- The 4d orbital can accommodate up to 10 electrons. In xenon’s electron configuration, it contains 10 electrons.
- The 5p orbital can accommodate up to 6 electrons, which is the case for xenon.
Combining all these subshells, we get the complete electron configuration for xenon as [Kr] 5s2 4d10 5p6. This configuration indicates that xenon has a full valence shell, making it an inert, or noble, gas. It is important to note that noble gases tend not to form compounds readily due to their stable electron configurations.
Understanding the electron configuration notation for xenon allows us to appreciate its chemical properties and how it interacts with other elements in various chemical reactions.
Significance Of Xenon’s Electron Configuration
Xenon’s electron configuration is significant in understanding its chemical properties and behavior. This arrangement of electrons in its energy levels determines its stability and reactivity, making xenon a valuable element in various applications such as lighting and medical imaging.
Role Of Electron Configuration In Chemical Reactivity
Applications Of Xenon’s Electron Configuration
The significance of Xenon’s electron configuration lies in its unique arrangement of electrons, which plays a crucial role in determining its chemical reactivity and various applications. Xenon, a noble gas, has an electron configuration of 2-8-18-18-8. This stable electron arrangement makes Xenon highly unreactive and inert, giving rise to several fascinating properties.
Role Of Electron Configuration In Chemical Reactivity
Xenon’s electron configuration of 2-8-18-18-8 contributes to its limited chemical reactivity. Noble gases like Xenon have completely filled electron shells, making them highly stable and unlikely to form chemical bonds with other elements. This stability arises from the fact that the outermost electron shell, also known as the valence shell, is completely filled with electrons. As a result, Xenon does not readily gain or lose electrons to achieve a more stable electron configuration, making it relatively inert in most chemical reactions.
However, despite its general unreactivity, Xenon can participate in certain chemical reactions under specific conditions. For example, when exposed to powerful reducing agents, such as fluoride ions, Xenon can form compounds like noble gas fluorides. These reactions occur due to the unique ability of Xenon’s electron configuration to accommodate extra electrons in its valence shell and form temporary bonds. The ability of Xenon to exhibit limited reactivity while maintaining its stable electron configuration makes it a valuable element in various applications.
Applications Of Xenon’s Electron Configuration
- Lighting: Xenon is widely used in light bulbs due to its stable electron configuration, which allows it to emit intense, bright light when an electric current is passed through it. This property makes Xenon lights ideal for automotive headlights, high-intensity discharge (HID) lamps, and photography flash units.
- Medical Imaging: Xenon’s electronic configuration also applies to medical imaging techniques such as magnetic resonance imaging (MRI) and computed tomography (CT) scans. In MRI, Xenon atoms are used as contrast agents to enhance the visibility of certain organs and tissues. In CT scans, patients can inhale Xenon gas to provide clearer images of the lungs.
- Chemical Analysis: Xenon’s unique electron configuration makes it advantageous in analytical chemistry. It can be used as a probe to study the behavior of atoms and molecules in various chemical reactions and environments. Its stable electron configuration allows it to serve as an inert marker or indicator in spectroscopy experiments, providing valuable insights into intermolecular interactions.
- Ion Propulsion: Xenon’s electron configuration is also suitable for space propulsion systems. In ion thrusters, Xenon atoms are ionized and accelerated using electric fields, producing high exhaust velocities. These thrusters offer efficient propulsion with low fuel consumption, making them ideal for long-duration space missions.
These are just a few examples of how the electron configuration of Xenon influences its properties and applications. By understanding the role of electron configuration, scientists can harness the unique characteristics of Xenon for various technological advancements and scientific investigations.
Xenon Electron Configuration Variations
Xenon, a noble gas in Group 18 of the periodic table, exhibits variations in its electron configuration. Understanding these variations is crucial to comprehending xenon’s behavior and properties. Let’s explore the intricacies of xenon’s electron configuration, exploring its isotopes and their effects.
Isotopes Of Xenon
Xenon has a total of nine naturally occurring isotopes, with atomic masses ranging from 124 to 136. The most abundant isotopes include xenon-128, xenon-129, and xenon-130. These isotopes differ in the number of neutrons, leading to variations in their physical and chemical properties.
Effects Of Isotopes On Electron Configuration
Isotopes of xenon impact its electron configuration by altering the number of neutrons in the nucleus, leading to variations in the energy levels of the electrons. This results in different arrangements of electrons in the outermost shell, influencing the reactivity and chemical behavior of xenon isotopes.
Exceptions In Xenon’s Electron Configuration
While xenon’s electron configuration typically follows the pattern of its atomic number and fills in the orbitals sequentially, certain exceptions are worth exploring. These exceptions, known as excited states, occur when electrons occupy higher energy levels than conventionally expected.
Explanation Of Excited States
Excited states in xenon’s electron configuration arise when an electron is promoted from its ground state to a higher energy level. This can occur when the energy supplied to the xenon atom exceeds the energy required for an electron to occupy an excited state orbital. The phenomenon of excited states allows xenon to exhibit unique chemical properties and participate in diverse chemical reactions.
Examples Of Excited States In Xenon
To further illustrate the concept of excited states in xenon, let’s explore a few examples:
-
- Excited State: 5s^24d^105p^65d^16s^2
This excited state occurs when a 6s electron is promoted to a higher energy level, occupying the 5d orbital instead of the 6s^2 orbital.
-
- Excited State: 5s^24d^105p^65d^25f^146s^2
In this case, a 6s electron is promoted twice to the 5d and 5f orbitals, resulting in an excited state configuration.
-
- Excited State: 5s^24d^105p^65d^105f^146s^25p^6
Here, multiple promotions of electrons to higher energy levels lead to a complex arrangement with electrons occupying the 5f and 6s orbitals.
These examples demonstrate that xenon’s electron configuration can deviate from the expected ground state arrangement due to the presence of excited states. These excited states play an essential role in xenon’s reactivity and allow it to form unique chemical compounds.

Experimental Determination Of Xenon’s Electron Configuration
Discover the experimental determination of xenon’s electron configuration, shedding light on its unique orbital filling sequence. This significant research contributes to understanding xenon’s chemical properties and behavior in various compounds and environments. Gain insights into the intricate arrangement of electrons within xenon’s energy levels.
Methods Used In Electron Configuration Determination
Studies And Research On Xenon’s Electron Configuration
Experimental methods are crucial in determining the precise arrangement of electrons within an element, like Xenon (Xe) ‘s atomic orbitals. Scientists have shed light on Xenon’s electron configuration by using various techniques and conducting thorough research. These studies provide essential insights into the behavior and properties of Xenon, such as its chemical reactivity and bonding abilities.
Methods Used In Electron Configuration Determination
Determining the electron configuration of an element like Xenon involves employing different experimental techniques. These methods enable researchers to understand the arrangement of electrons in the element’s orbitals, revealing valuable information about its chemical behavior and characteristics.
Studies And Research On Xenon’s Electron Configuration
Researchers have dedicated considerable effort to studying and researching Xenon’s electron configuration. These investigations have provided insights into the element’s behavior, chemical reactions, and bonding properties, helping to expand our understanding of this noble gas and its role in various fields of science and technology.
Some of the commonly used methods for determining the electron configuration of Xenon include:
- Electron Paramagnetic Resonance (EPR): This spectroscopic technique measures the magnetic properties of unpaired electrons in a substance, providing valuable information about their location and arrangement within the atom. By analyzing Xenon’s EPR spectrum, scientists can determine its electron configuration.
- Photoelectron Spectroscopy (PES): PES involves irradiating Xenon atoms with high-energy photons, causing their electrons to be ejected. By measuring the kinetic energy of these ejected electrons, scientists can determine their initial energy levels and, subsequently, the electron configuration of Xenon.
- X-ray Crystallography: This method involves bombarding a Xenon-containing crystal with X-rays and analyzing the resulting diffraction pattern. By examining the distribution of electron density in the crystal, scientists can infer the electron configuration of Xenon.
- Synthesis and Chemical Reactions: Studying the chemical reactions of Xenon compounds and observing their behavior offers valuable clues about the element’s electron configuration. By examining how Xenon forms bonds and interacts with other elements, scientists can gain insights into its electronic structure.
These methods, along with numerous studies and research projects, have contributed significantly to unraveling the precise electron configuration of Xenon. As a result, scientists have enhanced their understanding of this noble gas, its unique properties, and its importance in various scientific disciplines. Google Maps.
Frequently Asked Questions For Xenon Electron Configuration
How Do You Write The Electron Configuration For Xenon?
The electron configuration for xenon is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6.
Why Is The Electron Configuration Of Xenon 2 8 18 18 8?
The electron configuration of xenon is 2 8 18 18 8 because it has 54 electrons distributed among its energy levels.
What Element Is 1s2 2s2 2p5?
The element with the electron configuration 1s2 2s2 2p5 is fluorine, a member of the halogen group.
What Element Is 1s22s22p3?
The element with the electron configuration 1s22s22p3 is phosphorus (P).
Conclusion
Understanding the electron configuration of xenon is crucial for understanding its chemical properties. With its stable configuration and full outer shell, xenon exhibits low reactivity. This knowledge is valuable for various applications in industry and technology. By grasping the electron configuration of xenon, we can unlock its potential in many fields.