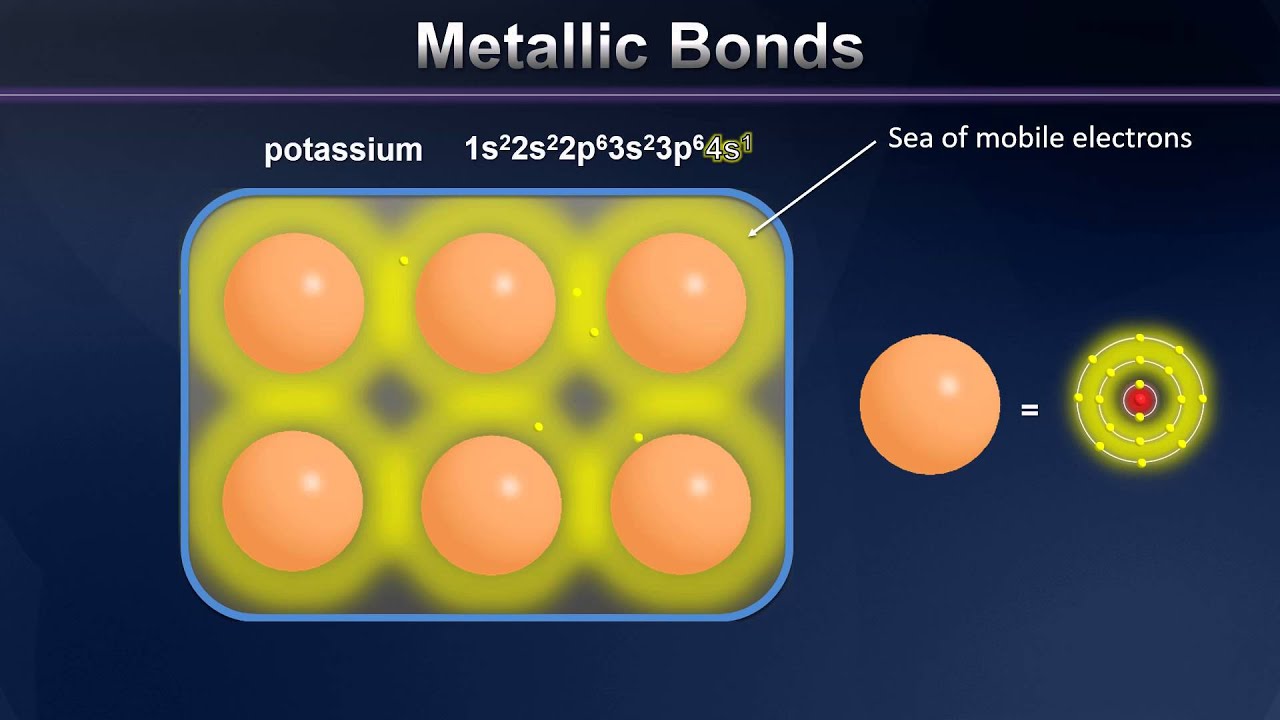
The metallic bond is the attraction between positive metal ions and delocalized electrons. In a metallic bond, positive metal ions are attracted to delocalized electrons, forming a strong bond.
This type of bonding allows metals to be conductive and malleable, making them essential for various industries such as construction, electronics, and manufacturing. Metallic bonds also contribute to metals’ high melting and boiling points and their ability to exhibit luster and conductivity.
Understanding metallic bonding is crucial in comprehending the unique properties and behavior of metals in different applications.

The Basics Of Metallic Bonding
Metallic bonding is a fundamental concept in chemistry that explains how metal atoms form strong bonds by sharing their outer electrons. This type of bonding allows metals to have unique properties like conductivity and malleability. It plays a crucial role in understanding the behavior and characteristics of metals.
Metallic bonding is a fundamental concept in chemistry, explaining how metals form strong connections with each other to create the unique properties we associate with metal. This type of bonding occurs when valence electrons, the outermost electrons of an atom, are free to move throughout a lattice of positive metal ions. Under these mobile electrons’ influence, metals exhibit electrical conductivity, malleability, and ductility. Understanding the basics of metallic bonding is crucial to comprehend the behavior and characteristics of metals.
What Is Metallic Bonding?
Metallic bonding refers to the chemical bonding that occurs between metal atoms. Unlike other types of bonding, such as ionic or covalent, metallic bonding does not involve the sharing or transferring of electrons between atoms. Instead, in metallic bonding, the valence electrons are delocalized or detached from the individual metal atoms, forming a “sea” of freely moving electrons that surround the metal ions. This unique arrangement allows the metal ions to pack closely together in a lattice structure while the free electrons are spread throughout the entire lattice. The positive metal ions, held in place by their attractive forces with the surrounding electrons, create a strong metallic bond that gives metals their characteristic properties.
How Does Metallic Bonding Work?
To better understand how metallic bonding works, let’s break it down into two key components: the metal lattice and the mobile electrons. The metal lattice is a repetitive three-dimensional arrangement of metal atoms or ions. This lattice structure is held together by strong electrostatic forces between the positive metal ions and the negatively charged sea of electrons. The regular pattern of the lattice allows for efficient close-packing of the metal ions, leading to a solid and dense metallic structure. The mobile electrons play a crucial role in metallic bonding. As mentioned earlier, these electrons are not bound to any specific metal atom but are free to move throughout the entire lattice. They create a kind of electron cloud that surrounds the metal ions. Due to their mobility, these electrons can conduct electricity and heat, as well as transmit mechanical forces within the metal structure. The ability of metals to conduct electricity is a direct result of this unique arrangement of mobile electrons. Furthermore, the presence of mobile electrons also influences the physical properties of metals. For example, metals are malleable and ductile because the mobile electrons can easily move around and adjust to the deformation of the lattice when subjected to external forces. This characteristic allows metals to be shaped into various forms, such as wires or sheets, without breaking. In conclusion, metallic bonding is a fascinating phenomenon that underlies the distinctive properties of metals. The delocalized nature of valence electrons allows metals to form a strong bond, creating an array of intriguing characteristics such as electrical conductivity, malleability, and ductility. Understanding metallic bonding is crucial in fields like materials science and engineering as it enables scientists and engineers to harness the unique properties of metals for practical applications.
Properties Of Metallic Bonding
Metallic bonding is a type of chemical bonding that occurs between metal atoms. It is responsible for giving metals their unique properties. Understanding the properties of metallic bonding can help us appreciate why metals are so vital in various fields, from electrical conductivity to structural applications. Let’s explore some of the remarkable properties of metallic bonding.
High Electrical Conductivity
Metallic bonding is renowned for its excellent electrical conductivity. This property allows metals to conduct electricity efficiently, making them essential in various electrical and electronic devices. In metallic bonding, the outer electrons of metal atoms are loosely bound and are free to move within the metal lattice. This “sea of electrons” can easily carry electric charge, resulting in the high electrical conductivity observed in metals.
Ductility And Malleability
Metals are highly ductile and malleable, meaning they can be stretched into thin wires and shaped into various forms respectively. This property is a direct result of metallic bonding. The flexible nature of metallic bonds allows metal atoms to slide past one another without disturbing the overall lattice structure. As a result, metals can undergo extensive deformation without breaking or losing their strength.
Luster And Reflectivity
Another striking property of metallic bonding is the luster and reflectivity of metals. When light interacts with a metal’s surface, it is absorbed by the free electrons, causing them to vibrate and re-emit the light waves. This phenomenon, known as metallic luster, gives metals their shiny appearance. Additionally, the ability of metal surfaces to reflect light contributes to the high reflectivity observed in metallic materials.
The Structure Of Metallic Bonds
Metallic bonds are formed between atoms in metal elements, enabling them to share their outermost electrons. These bonds result in a unique structure, where positively charged metal ions are surrounded by a sea of delocalized electrons, giving metals their characteristic properties such as malleability and conductivity.
In chemistry, metallic bonds play a crucial role in holding metals together. Understanding the structure of metallic bonds can provide insights into the unique properties of metals, such as conductivity and malleability.
Crystal Lattice
The crystal lattice is a key component of metallic bonds. It is a repeating three-dimensional pattern formed by the arrangement of metal atoms in a solid metal. Imagine a perfectly organized stack of marbles, each representing an atom. The crystal lattice gives metals their characteristic crystal structure, with a regular arrangement of atoms that extends indefinitely.
Within this crystal lattice, metal atoms are tightly packed together in an orderly manner. This proximity allows for the efficient sharing of valence electrons, which brings us to the next essential feature of metallic bonds.
Delocalized Electrons
Delocalized electrons are another critical aspect of metallic bonds. Unlike other types of chemical bonding, where electrons are bound to specific atoms, metallic bonds involve the sharing of valence electrons among all the atoms in the crystal lattice.
In a metal, the valence electrons are loosely held by their respective atoms, allowing them to move freely throughout the lattice. This delocalization gives metals their high electrical and thermal conductivity. When a voltage or heat is applied, these mobile electrons can easily flow and transfer energy.
| Key Elements of Metallic Bonds | |
|---|---|
| Crystal Lattice | Metal atoms arranged in a repeating, three-dimensional pattern |
| Delocalized Electrons | Valence electrons shared among all metal atoms in the lattice |
In summary, the structure of metallic bonds is characterized by a crystal lattice arrangement of metal atoms and the presence of delocalized electrons. This unique bonding mechanism gives rise to the extraordinary properties of metals, making them essential in various industries and everyday applications.
Formation Of Metallic Bonds
Metallic bonds are formed when positively charged metal ions are surrounded by a sea of delocalized electrons. These bonds contribute to metals’ unique properties, such as conductivity and malleability.
Metal Atoms And Ionic Bonds
Role Of Valence Electrons
The formation of metallic bonds is a fascinating process that occurs between metal atoms. Unlike other types of chemical bonding, such as ionic bonds or covalent bonds, metallic bonds involve the sharing of electrons among a vast number of metal atoms. This bonding type gives metals their unique properties, such as malleability, high conductivity, and luster.
Metal Atoms And Ionic Bonds
When it comes to understanding metallic bonds, it is essential to examine the structure of metal atoms and their behavior in bonding. Metal atoms have loosely held valence electrons, which are the electrons found in the outermost energy level of an atom. Unlike nonmetals, which tend to gain or lose electrons to achieve a stable electron configuration through ionic or covalent bonding, metals conduct electrons more freely due to the nature of their valence electrons.
In ionic bonds, metal atoms lose valence electrons to form positively charged ions known as cations. These cations then bond with negatively charged ions, or anions, forming an ionic compound. The electrostatic attraction between the cations and anions leads to the formation of a crystal lattice structure. This type of bonding is typical in compounds like sodium chloride (NaCl) or magnesium oxide (MgO), where the valence electrons completely transfer from one atom to another.
Role Of Valence Electrons
However, in the case of metallic bonding, metal atoms do not lose or gain electrons entirely but rather share them. The valence electrons move freely among the metal atoms, creating delocalized electrons surrounding a lattice of positively charged metal ions. This electron sharing produces a cohesive force that holds the metal atoms together in a lattice structure. The more valence electrons available for sharing, the stronger the metallic bond.
The shared electrons in metallic bonds allow metals to conduct heat and electricity. The delocalized electrons’ movement enables a charge flow when a current passes through a metal. The mobility of these electrons is also responsible for metals’ malleability, as they can easily slide past one another without breaking the metallic bond.
In conclusion, the formation of metallic bonds involves the sharing of valence electrons among metal atoms. This delocalization of electrons creates a unique bond that results in metals’ remarkable properties, making them indispensable in various fields such as engineering, construction, and electronics.
Examples And Applications Of Metallic Bonding
Metallic bonding occurs between metal atoms within a solid structure. It is characterized by the sharing of delocalized electrons, which allows metals to possess unique properties such as high electrical conductivity, thermal conductivity, and malleability. Let’s explore some examples and applications of metallic bonding in various aspects of our everyday lives!
Metals In Everyday Life
Metallic bonding plays a vital role in the functionality of numerous objects and materials we encounter on a daily basis. Here are some examples of how metallic bonding is prevalent in our everyday lives:
- Metallic electrical wires: Metals’ high electrical conductivity, attributed to metallic bonding, makes them ideal for use in electrical wires, ensuring efficient electricity transmission.
- Utensils and cookware: Many of our kitchen utensils and cookware, such as stainless steel cutlery and aluminum pans, utilize metallic bonding. This bonding provides durability and thermal conductivity, allowing for even heat distribution during cooking.
- Automobiles: Metallic bonding is essential for creating strong and lightweight materials used in automobile construction. It ensures structural integrity and enhances fuel efficiency.
- Building materials: Materials like steel and iron, which undergo metallic bonding, are commonly used in construction due to their strength and longevity.
Metallic Alloys
Metallic alloys are mixtures of two or more metals exhibiting metallic bonding. Alloys offer enhanced properties compared to pure metals, making them valuable for various applications. Let’s discover some examples:
- Stainless steel: This alloy, consisting mainly of iron, chromium, and nickel, is known for its corrosion resistance and durability. It finds application in kitchen appliances, cutlery, and even surgical instruments.
- Brass: A mixture of copper and zinc, brass possesses excellent thermal and electric conductivity, making it ideal for musical instruments, plumbing fittings, and decorative purposes.
- Aluminum alloys: Aluminum alloys, which combine aluminum with other metals like copper or magnesium, are widely used in aerospace engineering, automotive manufacturing, and construction due to their lightweight nature and high strength.
- Gold alloys: Gold alloys, created by blending gold with other metals like silver or copper, offer enhanced durability and versatility. They are commonly used in jewelry-making and dental applications.
With these examples and applications in mind, metallic bonding is essential for numerous aspects of our everyday lives, from wires’ electrical conductivity to the strength of construction materials. Its unique properties ensure that metals and alloys continue to play a crucial role in various industries and applications.
Strengths And Limitations Of Metallic Bonding
Metallic bonding is a type of chemical bonding that occurs between metal atoms. It is known for its unique properties, which give metals their characteristic qualities. However, metallic bonding has strengths and limitations like any other bonding type.
Strengths Of Metallic Bonding
Metallic bonding offers several strengths that make it a crucial factor in the properties and behaviors of metals. These strengths include:
- High electrical conductivity: Metallic bonds allow the easy movement of electrons, making metals excellent conductors of electricity.
- High thermal conductivity: Similarly, the free movement of electrons in metallic bonds enables metals to conduct heat efficiently.
- Malleability and ductility: The arrangement of positive metal ions embedded in a sea of delocalized electrons allows for the layers of metal atoms to slide over one another. This property makes metals malleable, enabling them to be hammered into thin sheets or drawn into thin wires without breaking.
- High melting and boiling points: The strength of metallic bonds requires significant energy to break, leading to high melting and boiling points in metals.
- Luster: The delocalized electrons in metallic bonding are responsible for the reflective and lustrous appearance of metals.
Limitations Of Metallic Bonding
Despite its strengths, metallic bonding also has certain limitations. These limitations include:
- Brittleness: Some metals, especially those with smaller metallic radii and stronger metallic bonds, can be brittle and prone to fracture.
- Non-directionality: Metallic bonds are not directional, meaning that the arrangement of metal atoms in a crystal lattice is not influenced by specific bonding angles or directions. This results in a lack of structural diversity compared to other bond types.
- Reactivity: Metallic bonds can lead to increased reactivity, as the delocalized electrons can readily interact with other substances, resulting in corrosion and oxidation of the metal.
In summary, metallic bonding offers unique strengths such as high electrical and thermal conductivity, malleability, and high melting points. However, it also has limitations that include brittleness, non-directionality, and increased reactivity. Understanding these strengths and limitations helps us comprehend the properties and applications of metals in various fields.
Comparing Metallic Bonding With Other Types Of Bonding
Metallic bonding distinguishes itself from other types of bonding, such as ionic or covalent, by sharing electrons among a lattice of metal atoms. This unique arrangement results in metals’ characteristic properties, such as malleability, conductivity, and high melting points.
Ionic Bonding
Ionic bonding is a type of chemical bonding that occurs between atoms when there is a significant electronegativity difference between them. In this type of bonding, one atom gains electrons to become negatively charged (anions), while another atom loses electrons to become positively charged (cations). The resulting electrostatic attraction between the positively and negatively charged ions creates a strong bond. Ionic bonds are typically found between metals and nonmetals, such as the bond between sodium (Na) and chlorine (Cl) in sodium chloride (NaCl), commonly known as table salt.
Covalent Bonding
Covalent bonding is another type of chemical bonding that occurs when two atoms share electrons to achieve a stable electron configuration. Unlike ionic bonding, there is no significant electronegativity difference between the atoms involved in covalent bonding. Instead, the atoms share electron pairs in order to fill their valence shells. Covalent bonds are typically found between nonmetals and nonmetals. Examples of covalent bonds include the bond between hydrogen (H) atoms in hydrogen gas (H2) and the bond between oxygen (O) atoms in water (H2O).
Metallic Bonding
Metallic bonding, on the other hand, is unique because it occurs exclusively between metal atoms. In this type of bonding, the valence electrons of metal atoms detach from their parent atoms and form a “sea” of delocalized electrons that surround the metal cations. This electron sea allows for the free movement of electrons throughout the metal lattice, giving metals their unique properties such as high electrical conductivity and malleability. Unlike ionic and covalent bonds, metallic bonding does not involve the sharing or transferring of electrons between atoms. Instead, the electrons are shared collectively by all the metal atoms in the lattice. Google Maps.
Frequently Asked Questions On Metalic Bond
What Is A Metallic Bond In Simple Terms?
A metallic bond is the force that holds metal atoms together in a solid. It occurs when electrons are shared among the atoms, creating a strong and flexible structure. This bond is what gives metals their characteristic properties like conductivity and malleability.
Why Are Metallic Bonds So Strong?
Metallic bonds are super strong due to the sharing of electrons by metal atoms. This creates a sea of delocalized electrons, which leads to the cohesive forces that hold the metal together. These bonds allow metals to have their characteristic properties like high melting points and good electrical conductivity.
What Is The Difference Between A Covalent Bond And A Metallic Bond?
A covalent bond involves sharing electrons between atoms, creating a strong bond. On the other hand, a metallic bond occurs when a metal atom donates electrons to a shared electron sea.
Are Metallic Bonds Malleable?
Yes, metallic bonds are malleable.
Conclusion
The metallic bond is a powerful force that holds metal atoms together, allowing them to conduct electricity and heat. Its unique properties make metals invaluable in various industries and everyday life. Understanding the nature of metallic bonds is crucial in designing and producing strong, durable, and functional materials.
By delving into the intricacies of this bonding mechanism, scientists and engineers can continue to innovate and improve our world. Harnessing the potential of metallic bonds will undoubtedly shape the future of technology and manufacturing.