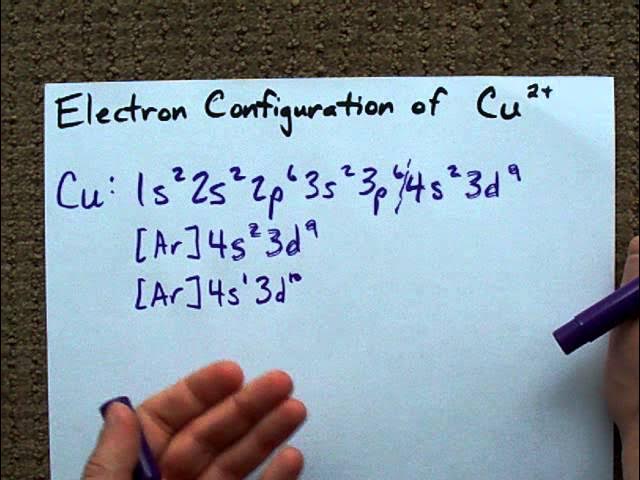
Copper’s electron configuration is [Ar] 3d10 4s1. This unique configuration is due to its partially filled d orbital.
Copper is a transition metal with atomic number 29. Its electron configuration showcases the arrangement of electrons in its orbitals. In the case of Copper, the 3d orbital is filled with 10 electrons, and the 4s orbital has 1 electron, resulting in a stable configuration.
This configuration contributes to Copper’s various oxidation states and its ability to form colorful compounds. Understanding copper’s electron configuration is essential in predicting its chemical behavior and properties. Let’s delve deeper into the significance of Copper’s electron arrangement and how it impacts its role in chemical reactions and industrial applications.

Credit: www.pw.live
The Importance Of Electron Configuration In Chemistry
The electron configuration of copper is an essential concept in chemistry as it determines its chemical behavior and properties. Understanding the arrangement of electrons in copper helps predict its reactivity and bonding patterns, which is crucial in various chemical reactions and applications.
The importance of electron configuration in chemistry cannot be overstated. Understanding the arrangement of electrons in an atom is crucial to comprehending how different elements interact with one another and how they form chemical bonds. This knowledge provides valuable insights into the unique properties and behaviors of each element, guiding scientists in everything from material design to pharmaceutical development.
Basic Concept Of Electron Configuration
“` Electron configuration refers to the distribution of electrons in the energy levels, or shells, of an atom. This arrangement is represented using a standardized notation to express the number of electrons in each energy level and the specific sublevels they occupy. For example, the electron configuration of copper is 1s22626210. This notation reveals the presence of unfilled d-orbitals in the outermost energy level of copper, impacting its chemical behavior.
Significance In Determining Chemical Properties
“` The arrangement of electrons directly influences an element’s chemical properties. Elements with the same electron configuration often exhibit similar chemical behaviors because their outermost energy levels have the same number and arrangement of electrons. For instance, elements in the same group of the periodic table share similar electron configurations and display analogous chemical properties. Understanding electron configuration allows chemists to predict how different elements will react with each other and form compounds, unlocking a deeper understanding of the fundamentals of chemical bonding and reactivity. This insight is invaluable in fields ranging from material science to environmental chemistry and beyond.
Overview Of Copper’s Electron Configuration
Copper’s electron configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s1. This arrangement indicates the distribution of electrons in copper’s orbitals, defining its chemical properties and stability. Due to its high electrical conductivity and corrosion resistance, the unique 3d10 configuration allows for versatile applications in various industries.
Explanation Of Electron Configuration
Copper is an intriguing element with a unique electron configuration. The electron configuration of an atom describes the arrangement of electrons within its orbitals. The electron configuration is frequently represented as [Ar]3d^104s^1 for copper. Let me break it down for you simply – copper has 29 electrons in total, and the electron configuration shows how these electrons are distributed among different energy levels and sublevels.
Relationship To Copper’s Position In The Periodic Table
Copper’s electron configuration is connected to its position in the periodic table. As you probably know, the periodic table is organized based on atomic number, representing the number of protons in an atom’s nucleus. Copper has an atomic number of 29, which places it in the fourth row of the periodic table. Being in this row signifies that copper has four energy levels, or electron shells, available for electron distribution.
Anomaly In Copper’s Electron Configuration
Copper’s unique electron configuration deviates from the typical pattern, with one electron moving from the 4s orbital to fill the 3d orbital. This anomaly results in Copper’s electron configuration being [Ar] 3d10 4s1 rather than [Ar] 3d9 4s2.
Introduction
Copper, a well-known metal with a stunning reddish-brown hue, possesses an unexpected electron configuration that deviates from the norm. This deviation, often referred to as an anomaly, has piqued the curiosity of scientists and chemists for many years. In this article, we will delve into the discussion on Copper’s unexpected electron configuration, unraveling the mystery behind this fascinating phenomenon.
Discussion On Copper’s Unexpected Electron Configuration
Copper’s electron configuration is typically represented as [Ar] 3d10 4s1. However, it is intriguing that it does not follow the expected pattern based on conventional electron-filling rules.
Normally, when electrons occupy the different energy levels in an atom, they fill the orbitals in a specific order. According to the Aufbau principle, the 4s orbital is expected to fill before the 3d orbital due to its lower energy level. However, in the case of copper, an exception occurs when one electron from the 4s orbital moves to the 3d orbital.
This anomaly in Copper’s electron configuration can be attributed to the stability achieved by having a full or half-full d orbital. By moving the electron from the 4s orbital to the 3d orbital, Copper achieves a half-filled 3d orbital which results in increased stability.
To better understand this anomaly, let’s break down Copper’s electron configuration:
1. Noble Gas Core:
The electron configuration starts with the noble gas core, which represents the electron configuration of the preceding noble gas. In the case of Copper, the noble gas core is represented by [Ar], indicating that Copper’s electron configuration is built upon the electronic structure of Argon.
2. Filling the 3d Orbital:
Next, we look at the 3D orbital. Copper’s 3D orbital can accommodate a maximum of 10 electrons. In Copper’s electron configuration, we observe that the 3D orbital is filled with all 10 electrons, following Hund’s rule. This filling order maximizes electron pairing, enhancing stability.
3. Filling the 4s Orbital:
Finally, we consider the 4s orbital. According to conventional filling rules, the 4s orbital is expected to be occupied before the 3d orbital. However, Copper deviates from this pattern by placing only one electron in the 4s orbital. This electron later moves to the 3d orbital to achieve a more stable configuration, despite the higher energy level of the 4s orbital.
This unconventional electron configuration of Copper truly showcases the complexity and exceptions within the realm of chemistry. Understanding these nuances helps us appreciate the remarkable nature of elements and the intricate electron arrangements that govern their behavior.
Theoretical Explanations For Copper’s Electron Configuration
Theoretical explanations play a crucial role in unraveling copper’s unique behavior. Deconstructing the theoretical underpinnings of copper’s electron configuration sheds light on the fundamental principles and rules that govern its arrangement of electrons.
Application Of Hund’s Rule
Copper’s electron configuration is governed by Hund’s rule, which states that electrons fill orbitals of the same energy level one by one before pairing up. This results in a configuration of 3d10 4s1 for copper, defying the expected 3d9 4s2 arrangement. Applying Hund’s rule provides a theoretical basis for understanding the anomalous electron configuration of copper, contributing to its unique properties and behavior.
Effect Of Exchange Energy
Exchange energy also influences copper’s electron configuration, leading to the 3d^10 4s^1 arrangement. The exchange energy favors a symmetrical distribution of electrons in the 3d orbitals, resulting in the filling of the 3d orbitals before the 4s orbital. This theoretical insight into the effect of exchange energy elucidates the rationale behind copper’s deviation from the expected electron configuration, offering a valuable theoretical understanding of its electronic structure.
Experimental Evidence Supporting Copper’s Electron Configuration
Copper’s electron configuration, 1s2 2s2 2p6 3s2 3p6 4s1 3d10, is supported by experimental evidence. This configuration accounts for its unique chemical properties and ability to form multiple oxidation states, making it essential in various industrial applications and biological processes.
Examples From Spectroscopic Studies
Spectroscopic studies provide direct evidence of Copper’s electron configuration.
Copper’s electron configuration is substantiated through various experimental methods.
Examples From Spectroscopic Studies
Spectroscopic studies provide direct evidence of Copper’s electron configuration.
Atomic emission spectroscopy reveals the energy levels of Copper ions.
X-ray photoelectron spectroscopy elucidates the electronic structure of Copper atoms.
UV-visible spectroscopy offers insights into Copper’s absorption properties.
Electron configuration data from these studies affirm Copper’s unique electronic arrangement.
Practical Implications Of Copper’s Electron Configuration
Copper’s electron configuration, 1s2 2s2 2p6 3s2 3p6 4s1 3d10, has practical implications in various fields. Its d10 configuration allows copper to exhibit unique properties such as excellent conductivity, malleability, and corrosion resistance, making it ideal for electrical wiring, plumbing systems, and architectural applications.
Additionally, copper’s electron configuration also plays a crucial role in determining its chemical reactivity and bonding behavior.
Impact On Copper’s Chemical Behavior
Copper’s electron configuration affects its chemical properties. It gives copper unique characteristics in reactions.
Relevance In Industrial Applications
Understanding the electron configuration is crucial. It impacts how copper is utilized in industries, as copper’s electron configuration dictates its behavior.
Comparison With Electron Configurations Of Other Transition Metals
Copper’s electron configuration is [Ar]3d^10 4s^1, setting it apart from other transition metals. Its unique electron arrangement contributes to its distinct properties and reactivity.
Contrast With Common Electron Configurations In Transition Metals
Copper, a transition metal, has an electron configuration of 1s2 2s2 2p6 3s2 3p6 3d10 4s1. When compared with other transition metals, such as chromium and zinc, copper stands out due to its unique half-filled d-orbital, resulting in a stable configuration.
This contrasts sharply with the more common stable electron configurations of other transition metals, as shown in the table below:
|
Transition Metal |
Electron Configuration |
|---|---|
|
Chromium |
1s2 2s2 2p6 3s2 3p6 3d5 4s1 |
|
Zinc |
1s2 2s2 2p6 3s2 3p6 3d10 4s2 |
Copper’s electron configuration sets it apart from the electron configurations of other transition metals. While most transition metals follow the pattern of filling the 4s orbital before the 3d orbital, copper deviates from this convention with an electron configuration of 3d10 4s1, resulting in its unique chemistry and properties.
Future Research Directions In Understanding Electron Configurations
Copper’s electron configuration, as an exception, is 1s2 2s2 2p6 3s2 3p6 4s1 3d10, deviating from the general pattern. Future research in understanding electron configurations must delve into the reasons behind this anomaly, shedding light on the underlying principles governing electron arrangement in elements with anomalous configurations.
The understanding of electron configurations, particularly that of copper, has been a subject of interest for researchers in the field of chemistry and materials science. As technology advances and new tools become available, it opens up avenues for further investigation into this important area within the realm of atomic structure. In this section, we will explore some promising areas for future research and potential discoveries that could contribute to a deeper understanding of electron configurations.
Areas For Further Investigation
There are several areas within the study of electron configurations that warrant further investigation. These areas include:
-
Quantum Mechanical Modeling Techniques
-
New or refined quantum mechanical modeling techniques can provide valuable insights into electron configurations. Researchers can explore novel computational methods to obtain more accurate predictions and explanations of the electron arrangements in copper and other elements. Developing advanced algorithms and models will play a crucial role in unraveling the complexities of electron configurations.
-
Experimental Spectroscopy
-
Advancements in experimental spectroscopy techniques offer immense potential to probe electron configurations. By incorporating cutting-edge instruments and methodologies, scientists can obtain detailed information about the energy levels and electron distributions within copper atoms. This data can be used to validate theoretical predictions and enhance our understanding of the underlying electron configuration principles.
-
High-Pressure Studies
-
Exploring how electron configurations change under extreme pressures holds great promise. High-pressure studies can shed light on the behavior of electrons in compressed environments and reveal unique electronic phenomena. By subjecting copper samples to different pressure conditions, researchers can investigate how the electron distribution adapts and how it affects the material’s properties.
-
Multielectron Excitations
-
Incorporating multielectron excitations into electron configuration models can lead to new insights. Traditional single-electron descriptions may not fully capture the complexity of electron distributions. By considering the interactions between multiple electrons, researchers can better understand the effects of electron-electron correlation and the collective behavior that emerges in materials like copper.
Potential Discoveries
Continued research into electron configurations could yield exciting discoveries, such as:
-
Uncovering novel electronic structures
-
Identifying unique properties associated with specific electron arrangements
-
Discovering mechanisms for controlling or manipulating electron distributions
-
Linking electron configurations to material properties and reactivity
-
Exploring new applications in fields such as catalysis, electronics, and energy storage
-
Developing a more comprehensive understanding of the electronic nature of copper and other elements
In conclusion, future research directions in understanding electron configurations, especially in the case of copper, offer exciting possibilities for uncovering fundamental principles and advancing our knowledge in the field of chemistry and materials science. By focusing on areas for further investigation and potential discoveries, scientists can push the boundaries of our understanding and pave the way for innovative applications based on the principles of electron configurations.
Frequently Asked Questions Of What Is The Electron Configuration Of Copper
What Is The Electron Configuration Of Copper?
Copper’s electron configuration is [Ar] 3d10 4s1, showing 29 electrons distributed among the orbitals. This arrangement results from the interaction of the electrons within the copper atom, providing stability and defining its chemical properties.
Why Is Copper’s Electron Configuration Unique?
Copper’s electron configuration stands out due to the stability afforded by a half-filled d orbital and the ability to accommodate an additional electron in the 4s orbital. This unique configuration contributes to copper’s diverse chemical behavior and widespread industrial use.
How Does Copper’s Electron Configuration Influence Its Properties?
Copper’s electron configuration governs its chemical and physical characteristics, contributing to its malleability, electrical conductivity, and corrosion resistance. The arrangement of electrons within its atomic structure facilitates the formation of various chemical bonds, enabling copper to fulfill a wide range of practical applications.
Conclusion
To summarize, copper’s electron configuration is [Ar] 3d10 4s1. This unique configuration allows copper to have exceptional properties and play a vital role in various applications. Understanding copper’s electron configuration helps us comprehend its chemical behavior and why it exhibits specific characteristics.
By delving into the intricacies of electron configurations, we can unlock the secrets of different elements and their functions in the world around us.