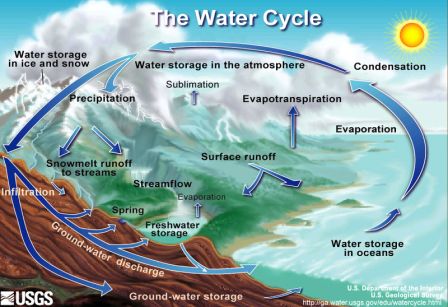
The Sublimation in water cycle occurs when water vapor directly transforms into ice without going through the liquid phase.
It involves various processes, including evaporation, condensation, precipitation, and sublimation. While evaporation converts liquid water to water vapor, sublimation is a process where water vapor directly transforms into ice or frost without becoming liquid. The water cycle, also known as the hydrological cycle, is the continuous water journey on Earth. This occurs when the temperature drops below freezing and the surrounding air is dry.
Sublimation is crucial in the water cycle as it contributes to snow and ice formation, affecting Earth’s climate, weather patterns, and water distribution. We will delve deeper into the phenomenon of sublimation and its significance in the water cycle.

Introduction To The Water Cycle
Introduction to the Water Cycle:
Definition And Overview
The Water Cycle, or the hydrological cycle, is the continuous water movement on, above, and below the Earth’s surface. This natural process involves converting water between its three states – liquid, solid, and gas – as it circulates through the atmosphere, oceans, land, and living organisms. It is an essential part of the Earth’s ecosystem, ensuring water availability for various biological processes.
Importance Of The Water Cycle
The Water Cycle plays a vital role in sustaining life on our planet. It not only regulates the distribution of water but also influences climate patterns, soil moisture, and the overall balance of ecosystems. Let’s explore the significance of the Water Cycle in more detail:
- Water Supply: The Water Cycle replenishes the Earth’s water supply by transferring water between different reservoirs. Through processes such as evaporation, condensation, and precipitation, freshwater availability for human consumption, plant growth, and maintaining aquatic habitats is ensured.
- Climate Regulation: The Water Cycle regulates the Earth’s climate by influencing temperature, humidity, and precipitation patterns. It helps distribute heat energy across the planet, moderating extreme temperatures and ensuring a stable climate for ecosystems to thrive.
- Nutrient Cycling: As water moves through the Water Cycle, it carries essential nutrients and minerals with it. This transports nutrients from land to oceans and vice versa, supporting the growth of plants, algae, and other organisms. It also helps break down organic matter, releasing nutrients back into the environment.
- Erosion and Sediment Transport: The Water Cycle is responsible for erosion and the transport of sediments across the Earth’s surface. It shapes landforms, such as valleys and canyons, through the continuous action of water flow, which gradually wears down rocks and carries the sediments to other locations.
- Water Conservation: The water cycle promotes water conservation and sustainability by recycling and redistributing water resources. It ensures a continuous flow of water, minimizing the waste of this precious resource and maximizing its use for various purposes.
Understanding Sublimation
Sublimation in the water cycle occurs when water molecules directly transform from solid ice to vapor without passing through the liquid stage. This process is essential for maintaining the balance of Earth’s ecosystems and plays a crucial role in weather patterns and climate regulation.
Definition Of Sublimation
Sublimation is an important process in the water cycle where a solid substance, such as ice or snow, changes directly into water vapor without going through the liquid phase. It is one of the three main phase change processes, alongside evaporation and condensation. Sublimation occurs when the surrounding temperature and pressure are below the substance’s triple point, causing it to transition from a solid to a gas state. This unique phenomenon plays a significant role in various natural and scientific processes.
Examples Of Sublimation
There are several examples of sublimation that occur in our daily lives and within the natural world. Let’s take a look at a few:
- Dry Ice: One of the most common examples of sublimation is seen with dry ice. Dry ice, which is solid carbon dioxide, undergoes sublimation at normal atmospheric pressure. It transforms directly from a solid to a gas, producing the characteristic fog-like smoke seen at concerts and in certain special effects.
- Naphthalene Balls: Another example of sublimation is seen with naphthalene balls commonly used as mothballs. Over time, these solid balls gradually disappear as they undergo sublimation, turning directly into gas and releasing their distinctive odor in the process.
- Snow: Snow, a familiar form of solid water, can also undergo sublimation under certain conditions. When exposed to sunlight or dry air with low humidity, snow can transform directly into water vapor without melting into liquid water first.
- Frost on Windows: During cold winter mornings, you might notice the delicate frost patterns on your windows. This frost forms when water vapor in the air comes into contact with the cold glass surface. The water vapor changes directly into solid ice crystals in a process known as sublimation.
- Freeze-drying: The freeze-drying process used in food preservation is another practical application of sublimation. By freezing food and then placing it in a vacuum chamber, the frozen water content sublimes, removing moisture from the food while maintaining its taste, texture, and nutritional value.
Understanding sublimation and its various examples provides insight into the dynamic nature of the water cycle and the remarkable transformations that water undergoes in different states of matter. By recognizing these processes, we can better appreciate our natural world’s intricate balance and interconnectedness.
Sublimation In The Water Cycle
Sublimation is a process in the water cycle where ice turns directly into water vapor, bypassing the liquid state. It occurs when the temperature and pressure are not ideal for the formation of liquid water.
Explanation Of Sublimation In The Water Cycle
Sublimation in the water cycle is a fascinating natural process in which ice transforms directly into vapor without entering the liquid state. Unlike melting or evaporation, sublimation bypasses the liquid phase altogether. This exceptional transformation occurs when the surface molecules of ice gain enough energy to break free and become water vapor, converting ice directly to gas.
Sublimation primarily takes place in regions where temperatures are extremely cold, such as polar ice caps and high-altitude mountain ranges. These areas experience low atmospheric pressure and a lack of liquid water, making sublimation a key player in the water cycle by enabling the conversion of solid ice into water vapor.
Role Of Sublimation In Water Vapor
Sublimation plays a crucial role in forming water vapor, which is essentially water in its gaseous state. It contributes to the constant replenishment of water vapor in the atmosphere, which in turn contributes to cloud formation and precipitation. Through sublimation, the frozen water present in snow and ice fields can directly transition into water vapor, which then rises into the atmosphere.
Once in the atmosphere, water vapor can transport heat and energy across various regions, affecting weather patterns and climate. Additionally, water vapor acts as a greenhouse gas, retaining heat in the atmosphere and significantly regulating Earth’s temperature.
The sublimation process is especially critical in areas with scarce liquid water, such as deserts or regions experiencing drought. Without sublimation, these areas would have limited access to atmospheric moisture, hindering the chances of precipitation and exacerbating aridity.
Factors Affecting Sublimation
Sublimation, a crucial process in the water cycle, is affected by various factors. These factors include temperature, atmospheric pressure, and surface area, all influencing the rate at which a substance transitions from solid to gas without passing through the liquid phase.
Factors Affecting Sublimation Sublimation is a process in the water cycle wherein water changes from its solid state (ice) directly to its gaseous state (water vapor) without going through the liquid phase. This unique phenomenon is influenced by several factors that affect the rate and occurrence of sublimation. Understanding these factors can provide valuable insights into the water cycle dynamics and its environmental impact. This section will explore two significant factors in sublimation: temperature and pressure, surface area, and wind speed.
Temperature And Pressure
Temperature and pressure are closely linked and substantially influence the sublimation process. As the temperature increases, the rate of sublimation also tends to rise. This is due to the fact that higher temperatures provide more energy to the water molecules, causing them to move faster and escape the solid phase more readily. Similarly, lower pressures can enhance sublimation since they reduce the forces of attraction between water molecules, making it easier for them to transition from solid to gas. Changes in temperature and pressure can have significant effects on sublimation rates. For example, sublimation can occur faster in cold and arid regions with low atmospheric pressure, such as high-altitude mountain ranges. On the other hand, in warmer and more humid environments with high atmospheric pressure, the sublimation rate may be considerably slower.
Surface Area And Wind Speed
Another crucial factor influencing sublimation is the surface area of the solid ice or snow. A larger surface area allows for more water molecules to be exposed to the surrounding air, increasing the chances of sublimation. This is why powdered snow or crushed ice tends to sublimate more rapidly than compacted snow or large ice blocks. Additionally, the speed of the wind also has a significant impact on sublimation. Higher wind speeds promote quicker sublimation by removing the water vapor as it is released from the solid ice or snow. The moving air molecules effectively carry away the water vapor, preventing its reabsorption by the surrounding surfaces. Consequently, areas with strong and consistent winds experience more pronounced sublimation rates than regions with calm or stagnant air. In summary, temperature, pressure, surface area, and wind speed are all critical factors that affect sublimation in the water cycle. By understanding these factors and their interplay, we can gain a deeper understanding of the processes that drive the movement of water in its various forms.
Processes Involved In Sublimation
Sublimation is a fascinating process in the water cycle. It occurs when water molecules transition directly from a solid (ice) to a gas (water vapor) without undergoing the liquid phase. This unique phenomenon is crucial in the overall water cycle and significantly impacts weather patterns and climate. In this article, we will explore the processes involved in sublimation, focusing on evaporation, condensation, and deposition.
Evaporation
Evaporation is when liquid water transforms into water vapor by applying heat energy. When the sun’s rays reach bodies of water, such as lakes, rivers, and oceans, the water molecules gain energy and become more energetic. As a result, these molecules break free from the liquid surface and enter the gaseous state. The process of evaporation is crucial in the water cycle as it replenishes the atmosphere with water vapor, which eventually leads to the formation of clouds and precipitation.
Condensation
Condensation is the opposite process of evaporation, occurring when water vapor cools and transforms back into liquid water. As the atmosphere cools, the water vapor molecules lose heat energy and slow down. This causes them to come together and form tiny droplets, which become visible as clouds. The condensed water droplets can further combine to create larger droplets, ultimately leading to the formation of rain, snow, or other types of precipitation. Condensation is a crucial step in the water cycle as it allows water to return to the Earth’s surface and continue the cycle.
Deposition
Deposition is a specific type of sublimation that occurs when water vapor transitions directly into solid ice crystals or frost. This process typically occurs when the air temperature is below freezing point, and enough moisture is present in the atmosphere. When water vapor comes into contact with a cold surface, it loses heat energy rapidly and directly transforms into icy particles. These ice crystals or frost can accumulate on objects such as plants, trees, and windows, creating beautiful winter landscapes. Deposition plays a significant role in the water cycle by removing water vapor from the atmosphere and depositing it directly as solid ice.

Effects Of Sublimation
Sublimation, the process by which ice directly changes into vapor without melting into a liquid state, significantly affects various aspects of the water cycle. The occurrence of sublimation plays a crucial role in climate and weather patterns, as well as in glacial retreat.
Climate And Weather Patterns
Sublimation affects climate and weather patterns by reducing the amount of water available in the atmosphere as vapor. As ice changes directly into water vapor, it bypasses the liquid phase, decreasing overall humidity. This decrease in humidity can impact the formation of clouds and the occurrence of precipitation.
When sublimation occurs more frequently, such as in dry and cold regions like polar areas and high-altitude regions, it contributes to the creation of arid climates. These regions experience little rainfall due to the lack of water vapor in the atmosphere. On the other hand, in regions with higher humidity, sublimation can influence the onset and intensity of snowfall.
Glacial Retreat
Glacial retreat is another significant effect of sublimation. As ice directly changes into vapor through sublimation, glaciers lose mass and shrink over time. This process is particularly pronounced when sublimation rates exceed snow and ice accumulation. The effects of global warming and rising temperatures further contribute to increased sublimation rates, leading to significant glacial retreat.
Glacial retreat has far-reaching consequences, including rising sea levels, disrupted ecosystems, reduced freshwater resources, and altered weather patterns in glacier-dependent regions. The accelerating retreat of glaciers due to sublimation highlights the urgent need for understanding and mitigating the impacts of climate change.
Applications And Uses Of Sublimation
Sublimation plays a vital role in the water cycle. Solid ice directly converts into water vapor without going through the liquid phase. This process helps maintain the Earth’s water balance and contributes to weather patterns and precipitation.
Freeze-drying In Food Preservation
Sublimation is essential in several applications and industries, offering numerous benefits and uses. One of the notable applications of sublimation is in the process of freeze-drying, particularly in food preservation. Freeze-drying involves removing water from food products while preserving their texture, taste, and nutritional value. This technique is widely used to extend the shelf life of various food items, including fruits, vegetables, meats, and even dairy products. By subjecting the food to freezing temperatures, followed by a vacuum environment, the water content in the food undergoes sublimation, directly changing from the solid state (ice) to the gaseous state (water vapor) without passing through the liquid state. This process helps remove moisture effectively, preventing the growth of microorganisms and food spoilage.
Sublimation In Scientific Research
In the realm of scientific research, sublimation has proven to be a valuable technique with a range of applications. One of the prominent uses of sublimation is in sample preparation for microscopy and analysis. Sublimation is employed to create high-quality replicas of delicate biological specimens by converting solid substances, such as iodine, into vapor without the need for additional solvents. Moreover, sublimation is extensively employed in purifying and separating various substances. Sublimation purification is commonly used in the pharmaceutical industry to isolate and purify chemical compounds, ensuring their potency and effectiveness in medicinal formulations. Additionally, sublimation is utilized to synthesize novel materials, such as nanomaterials and porous substances, with unique properties and applications in various fields. In scientific research, sublimation is also applicable in the determination of vapor pressure, where the sublimation rate is measured to calculate the saturation pressure and other thermodynamic properties of a substance. This information is crucial for understanding the behavior of compounds under different conditions, aiding in the development of efficient industrial processes and environmental studies. Overall, sublimation demonstrates its significance and versatility in diverse areas such as food preservation and scientific research. Whether it is preserving the quality of food products or enabling advanced scientific investigations, sublimation proves to be an indispensable process with multifaceted applications. By harnessing the transformative power of sublimation, researchers and industries continue to explore innovative ways to enhance the quality of products and deepen our understanding of the natural world. Google Maps.
Frequently Asked Questions On Sublimation In Water Cycle
What Is Sublimation Process?
The sublimation process transforms a solid directly into a gas without passing through the liquid phase. It occurs when heat is applied to the solid, causing its particles to vibrate and escape as gas molecules. This process is commonly used in printing, where ink is converted into gas and bonded to materials like fabric or ceramics.
What Is The Sublimation Of The Condensation Cycle?
Sublimation in the condensation cycle is when a substance, like dry ice or frost, changes from a solid directly to a gas without going through the liquid phase. It is commonly used in freeze-drying and air-conditioning systems.
What Is The Sublimation Of Precipitation?
Sublimation of precipitation is when water vapor directly turns into ice crystals or frost without becoming a liquid.
What Is An Example Of Sublimation?
An example of sublimation is dry ice turning into a gas without melting into a liquid state.
Conclusion
Sublimation plays a crucial role in the water cycle. It transforms water vapor directly into ice without passing through the liquid phase. This phenomenon contributes to snow and ice formation on mountains, which later melts and feeds rivers and lakes.
Understanding sublimation helps us comprehend the intricate dynamics of the water cycle and its impact on our environment. Embracing this knowledge allows us to better appreciate the interconnectedness of Earth’s systems.