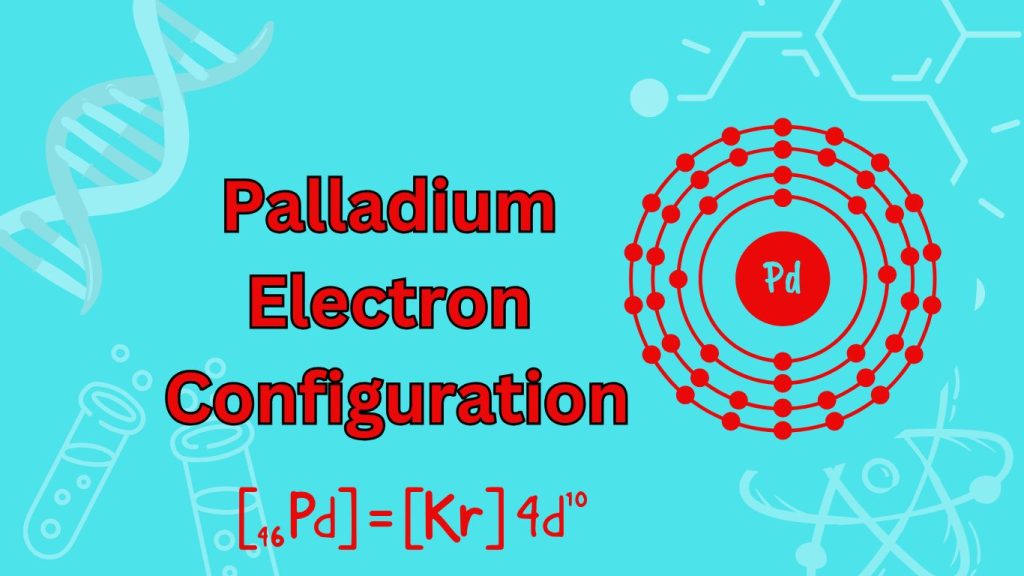
The Palladium electron configuration is [Kr] 4d10 5s2. Palladium is a transition metal belonging to the periodic table’s platinum group.
It has atomic number 46 and is known for its excellent catalytic properties and use in various industrial applications. With a full 4d orbital and two valence electrons in the 5s orbital, palladium exhibits stability and a wide range of chemical reactions.
Its electron configuration indicates the arrangement of electrons in its energy levels and orbitals, providing insight into its chemical behavior and properties.
What Is Palladium?
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a lustrous silver-white metal discovered in 1803 by William Hyde Wollaston, and it belongs to the platinum group of metals. Palladium is known for its remarkable catalytic properties, making it a vital element in various industrial applications. In this section, we will explore palladium’s atomic number and physical and chemical properties to gain a deeper understanding of this intriguing element.
Atomic Number
Palladium has an atomic number of 46, indicating the number of protons found in its nucleus. This property uniquely identifies palladium among the elements and is crucial in defining its chemical behavior and characteristics.
Physical And Chemical Properties
Palladium exhibits numerous intriguing physical and chemical properties contributing to its widespread utility. These properties include:
- High melting point
- Good ductility and malleability
- Excellent corrosion resistance
- Superior catalytic activity
These remarkable attributes make palladium essential in various industries, including automotive, electronics, and chemical manufacturing.
What Is Electron Configuration?
The electron configuration of palladium is [Kr] 4d10 5s0. In other words, it has 46 electrons distributed across its energy levels.
Definition
Electron configuration refers to the arrangement of electrons within an atom, specifically in its electron
shells or energy levels. Bold Text
Importance
Understanding electron configuration is crucial in comprehending an element’s chemical behavior. It provides
valuable insights into the stability and reactivity of atoms and can help predict their ability to form
chemical bonds. An atom tends to have a more stable electron configuration when its outermost electron shells
are either completely filled or empty. Bold Text
By knowing an element’s electron configuration, scientists can determine its position in the periodic table,
predict its chemical properties, and explain its behavior in various chemical reactions. This information is
essential in fields such as materials science, chemistry, and physics. Bold Text
Palladium Electron Configuration
Palladium (Pd) is a chemical element with atomic number 46 and is part of the platinum group of metals. In this section, we will explore the electron configuration of palladium and understand its ground state configuration and excited state configurations.
Ground State Configuration
The ground state electron configuration of palladium is written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f14 5d8. Let’s break it down:
- The first shell (1s) has 2 electrons.
- The second shell (2s, 2p) has 8 electrons.
- The third shell (3s, 3p, 3d) has 18 electrons.
- The fourth shell (4s, 4p, 4d, 4f) has 32 electrons.
- The fifth shell (5s, 5p, 5d) has 18 electrons.
Combining all these electrons gives us a total of 46, which corresponds to the atomic number of palladium.
Excited State Configurations
Palladium can also have various excited state configurations when its electrons are promoted to higher energy levels. These excited states are represented by changing the arrangement of the electrons. Here are a few examples:
- Excited State Configuration 1: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5 4f14 5d9
- Excited State Configuration 2: 1s2 2s2 2p6 3s2 3p6 4s2 3d9 4p6 5s2 4d10 5p6 4f14 5d9
- Excited State Configuration 3: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 5s2 4d10 5p6 4f14 5d10
These excited state configurations demonstrate the movement of electrons to higher energy levels, resulting in different arrangements within the electron cloud of palladium.
Understanding the electron configuration of palladium provides valuable insights into its chemical properties and reactivity. It plays a crucial role in explaining the variety of compounds and complexes that palladium can form, making it an important element in fields like catalysis and electronics.
Understanding Ground State Configuration
Palladium’s electron configuration in its ground state is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10. This arrangement of electrons in its orbitals reflects stability and minimal energy. Understanding the ground state configuration of palladium is vital in comprehending its chemical behaviors and properties.
Understanding Ground State Configuration
Explaining the Palladium Electron Configuration is crucial to understanding its ground state configuration. An atom is in its lowest energy state in the ground state, with electrons filling the orbitals according to specific rules. Understanding the electron distribution pattern in the ground state configuration is essential for comprehending the behavior and properties of Palladium. Let’s delve into a detailed explanation of this concept.
Explanation
H3html headings must be in HTML syntax
Electron Distribution Pattern
H3html headings must be in HTML syntax
Significance Of Ground State Configuration
The ground state configuration of an element is the arrangement of its electrons in the lowest energy levels or orbitals. It plays a crucial role in determining the properties and behavior of atoms. Understanding the significance of ground state configuration is essential in comprehending the characteristics of Palladium (Pd), a highly valuable chemical element.
Stability
The ground state electron configuration of Palladium is [Kr] 4d^10. This stable configuration exhibits a completely filled 4D orbital, contributing to its exceptional stability. Palladium is less likely to readily gain or lose electrons with a fully occupied electron shell, making it less reactive. This stability is a key factor in using Palladium in various industrial applications and as an important catalyst in chemical reactions.
Chemical Reactivity
Palladium’s ground state configuration affects its chemical reactivity. Although it is relatively stable, Palladium can still form compounds and participate in chemical reactions. Its partially filled 4D orbitals allow complexes with ligands or other elements to form. This ability to form coordination compounds makes Palladium versatile and useful in organic synthesis, catalysis, and numerous other chemical processes.
Beyond stability and chemical reactivity, the ground state configuration of Palladium influences its unique physical and electronic properties. These properties and stability contribute to this precious metal’s wide range of applications and significance in industries such as automotive, electronics, jewelry, and environmental technologies.
Electron Configuration Notation
The electron configuration notation for palladium involves listing the specific arrangement of its electrons in its atomic orbitals. This notation provides valuable information on the distribution of electrons in an atom.
The electron configuration notation is a concise and organized way to represent how electrons are distributed within an atom’s energy levels. It provides valuable insights into an element’s chemical properties and determines its placement on the periodic table. Understanding electron configuration notation is crucial for comprehending the behavior of elements and their interactions in chemical reactions.
Orbital Diagram
An orbital diagram is a visual representation of an atom’s electron distribution using boxes to represent orbitals and arrows to indicate electrons. Each box represents an orbital, which can hold a maximum of two electrons with opposite spins. The diagram provides a clear and intuitive picture of the electron arrangement within an atom, making it easier to understand and remember.
Noble Gas Configuration
The noble gas configuration represents an element’s electron configuration by using the electron configuration of the closest noble gas with a lower atomic number. Noble gases have stable electron configurations, making them useful reference points. By starting with the noble gas configuration, we can abbreviate the electron configuration notation and simplify it, especially for elements with many electrons.
For example, the electron configuration of palladium (Pd) can be expressed using the noble gas configuration of the previous element, which is krypton (Kr). Instead of writing the entire electron configuration from scratch, we can start with [Kr] (5s^24d^8) and then add the remaining electrons specific to palladium.
Exploring Excited State Configurations
Definition
At an excited state, an atom’s electron arrangement differs from its ground state configuration. This temporary state occurs when an electron gains energy and transitions to a higher energy level, causing fluctuations in the electron configuration.
Energy Level Transitions
Energy level transitions in an atom occur when an electron absorbs or emits energy. When an electron absorbs energy, it moves to a higher energy level, resulting in an excited state configuration. Conversely, when an electron emits energy, it transitions to a lower energy level, returning to its ground state configuration.
Factors Influencing Excited State Configurations
In studying palladium electron configuration, it is important to understand the factors that influence excited state configurations. These factors are crucial in determining palladium’s electronic structure and properties. By examining the effects of temperature and external stimuli, we can gain a deeper understanding of how excited state configurations are formed and their significance.
Temperature
The temperature at which a system is maintained can significantly impact the excited state configurations of palladium. At higher temperatures, the energy levels of the electrons increase, resulting in electron transitions to higher energy orbitals. This transition can lead to a rearrangement of the electron configuration, altering the electronic and chemical properties of palladium. On the other hand, lower temperatures tend to stabilize the electron configuration, minimizing the excitation of electrons to higher orbitals.
External Stimuli
External stimuli can also influence palladium’s excited state configurations. Various factors, such as light, pressure, and electric fields, can induce changes in the electronic structure of palladium. For instance, exposure to specific wavelengths of light can excite electrons to higher energy levels, leading to a modification in the electron configuration. Similarly, applying pressure or subjecting palladium to electric fields can alter the spatial distribution of the electrons, resulting in new excited state configurations.
Overall, understanding the factors influencing excited state configurations is crucial in comprehending the behavior and properties of palladium. By considering the effects of temperature and external stimuli, scientists can gain insights into how to control and manipulate the electronic structure of palladium, opening up possibilities for various applications in fields such as catalysis, electronics, and material science.
Applications Of Electron Configuration
Understanding an element’s electron configuration is crucial for uncovering its unique properties and applications. By analyzing the arrangement of electrons within an atom’s orbitals, scientists can predict how an element will interact with other substances, forming chemical bonds and driving important reactions. In this article, we will explore two key applications of electron configuration: chemical bonding and catalysis. Let’s dive in!
Chemical Bonding
Chemical bonding is the process of combining different elements to form stable compounds. The electron configuration of elements plays a vital role in determining how these bonds are formed. By examining the position of electrons in their respective orbitals, scientists can better understand the likelihood of elements forming ionic, covalent, or metallic bonds.
In ionic bonding, atoms transfer or share electrons to achieve a more stable electron configuration. Elements with a few valence electrons, such as sodium (Na) with a configuration of 2,8,1, readily give up their extra electron to achieve a stable octet. Meanwhile, elements like chlorine (Cl), with seven valence electrons, eagerly accept an extra electron, completing their outermost shell. This transfer creates an ionic bond, resulting in compounds like sodium chloride (NaCl), commonly known as table salt.
Covalent bonding, on the other hand, occurs when atoms share electrons to achieve a full outer shell. Elements like carbon (C) with four valence electrons can form multiple covalent bonds, creating complex compounds such as methane (CH4). The electron configuration reveals how atoms can complete their valence shell and form strong covalent bonds, enabling the formation of countless organic and inorganic substances.
Finally, metallic bonding, seen in metals like iron (Fe), relies on electrons’ ability to move freely within a lattice structure. Metals’ unique electron configuration allows their valence electrons to detach from individual atoms and form a “sea” of delocalized electrons. This electron mobility gives rise to metals’ excellent thermal and electrical conductivity, as well as their malleability and ductility.
Catalysis
Catalysis refers to the process in which a substance, known as a catalyst, increases the rate of a chemical reaction without undergoing permanent changes. The electron configuration of catalysts determines their ability to provide an alternate reaction pathway with lower activation energy, allowing reactions to occur more rapidly.
Transition metals, such as palladium (Pd), exhibit unique electron configurations that make them highly effective catalysts. Their partially filled d-orbitals enable them to accept and donate electrons during a reaction, facilitating the formation and breaking of chemical bonds. For example, palladium catalysts are crucial in automobile catalytic converters, converting harmful pollutants like carbon monoxide (CO) and nitrogen oxides (NOx) into less harmful substances.
The ability of palladium to adsorb and dissociate hydrogen gas (H2) makes it an essential catalyst in hydrogenation reactions, widely used in the production of pharmaceuticals, plastics, and fuels. The knowledge of palladium’s electron configuration enables scientists to design more efficient catalysts for these reactions, optimizing both cost and environmental impact.
Understanding the applications of electron configuration not only helps us grasp the behavior of elements but also opens paths to discover new materials and advance technological innovations. By utilizing this knowledge in various fields, from materials science to environmental engineering, scientists can make significant progress in addressing global challenges and improving our everyday lives.
Palladium’s Unique Electron Configuration
Due to its arrangement of electrons in energy levels, palladium’s electron configuration is unique. This rare configuration influences its chemical and physical properties, making palladium a highly sought-after element in various industries.
Palladium’s Unique Electron Configuration
Palladium, with its atomic number 46, exhibits a unique electron configuration contributing to its distinct properties and applications.
Explanation
Palladium’s electron configuration is [Kr] 4d^10. This means that it has 46 electrons arranged in different energy levels and sublevels. The electronic structure of palladium reflects its position in the periodic table and influences its chemical behavior.
Relationship To Properties
The electron configuration of palladium directly impacts its physical and chemical properties. For instance, the filled 4d shell contributes to palladium’s exceptional ability to absorb hydrogen, making it an essential component in catalytic converters. Additionally, completely filled d orbitals enhance its oxidation resistance, making it incredibly durable.
In summary, palladium’s electron configuration is key in determining its unique properties and applications, making it an intriguing element to study and utilize in various industrial processes.
Electronic Transitions In Palladium
Electronic transitions occur in palladium due to changes in the electron configuration. These transitions are crucial to the metal’s reactivity and electronic properties. Understanding Palladium’s electron configuration is essential for various catalysis and materials science applications.
Understanding palladium’s electronic transitions is crucial to its study. These transitions involve the movement of electrons between different energy levels, resulting in the absorption and emission of specific wavelengths of light. In this section, we will explore palladium’s absorption and emission spectra and gain insights into its fascinating electronic transitions.
Absorption Spectrum
The absorption spectrum of palladium provides valuable information about the wavelengths of light that are absorbed by the metal. As electrons transition from lower energy levels to higher ones, they absorb photons with specific energies corresponding to certain colors in the visible spectrum. This absorption is unique to each element and helps identify and characterize its electronic structure.
When exposed to various wavelengths, palladium selectively absorbs light at distinct energies. The electron configuration of palladium, where electrons occupy specific orbitals and energy levels, determines these absorbed wavelengths. As a result of these absorption processes, certain colors are absorbed while others are transmitted or reflected. Google Maps.
Emission Spectrum
The emission spectrum of palladium reveals the wavelengths of light that are emitted when electrons transition from higher energy levels to lower ones. When palladium is excited, for example, by heating or applying an electric field, electrons absorb energy and move to higher energy levels. As these excited electrons return to their ground state, they release energy as photons with specific energies.
The emission spectrum is unique to each element and can be used to identify and analyze palladium compounds. By measuring the emitted wavelengths, scientists can gain insights into the electronic structure of palladium and detect its presence in various samples.
Overall, the electronic transitions in palladium are essential for understanding its behavior and properties. The absorption and emission spectra provide valuable clues about the arrangement of electrons in the metal and offer a glimpse into the fascinating world of palladium’s electronic transitions.
Frequently Asked Questions For Palladium Electron Configuration
What Is The Electron Configuration Of Palladium?
The electron configuration of palladium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d8.
Why Is Palladium Electron Configuration 4d10?
The electron configuration of palladium is 4d10 due to its atomic structure and the filling of electron shells.
What Element Is 1s2 2s2 2p6 3s2 3p6 4s2 3d3?
The element with the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d3 is Vanadium (V).
What Element Has An Electron Configuration Of 1s 2 2s 2 2p 6 3s 2 3p 4?
The element with the electron configuration of 1s2 2s2 2p6 3s2 3p4 is sulfur (S).
Conclusion
Understanding the electron configuration of palladium is essential for grasping its chemical properties. Scientists can predict its reactions and behavior in various compounds by comprehending the energy levels and orbital arrangement. This knowledge is crucial for catalysis, electronics, and environmental remediation applications.
Exploring the electron configuration of palladium opens up numerous possibilities for scientific and technological advancements.

