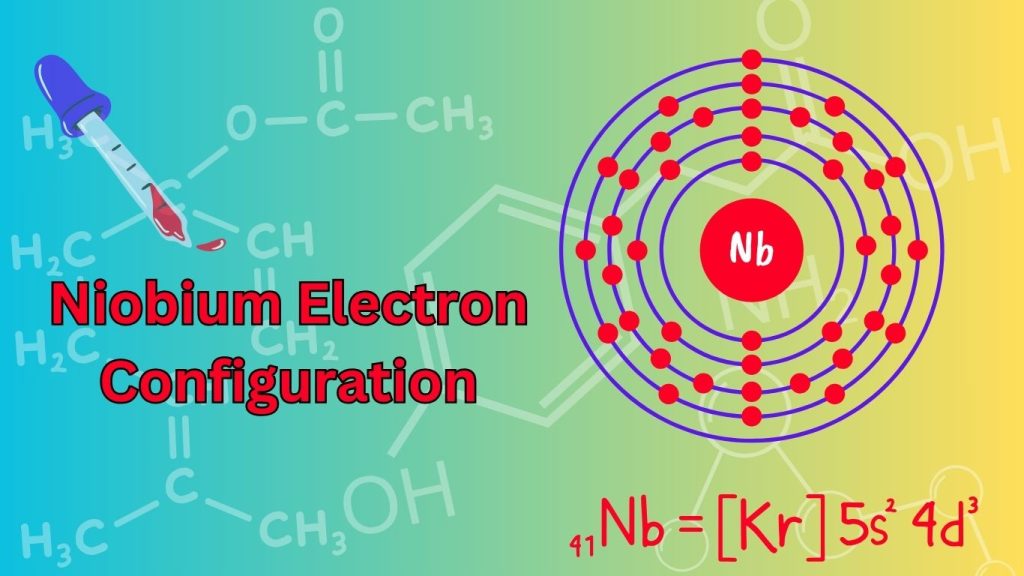
The Niobium Electron Configuration is [Kr] 5s2 4d3. Niobium is a chemical element with the symbol Nb and atomic number 41.
This transition metal belongs to the d-block and can be found in group 5 of the periodic table. Niobium’s electron configuration is denoted as [Kr] 5s2 4d3, indicating that it has 41 electrons in total. The noble gas krypton ([Kr]) represents the core electron configuration, followed by the valence electrons in the 5s and 4d orbitals.
Understanding niobium’s electron configuration is vital to understanding its chemical properties and role in various industrial and technological applications.
Learn more about Mercury Electron Configuration in the main guide.
What Is Niobium?
What is Niobium?
Niobium is a chemical element with the symbol Nb and atomic number 41. It is a lustrous, gray, ductile metal typically found in pyrochlore and columbite minerals. Niobium is widely used in various industries, including electronics, superconducting materials, and alloy manufacturing.
Atomic Number And Symbol
Niobium has an atomic number of 41 and is represented by the symbol Nb. It belongs to group 5 and period 5 in the periodic table, making it a transition metal.
Physical Properties
Niobium exhibits a range of fascinating physical properties:
- Metallic luster: Niobium has a characteristic metallic sheen.
- Ductility: It is highly ductile and can be stretched into thin wires.
- Melting point: Niobium has a high melting point of 2,468 degrees Celsius.
- Superconductivity: At low temperatures, niobium becomes superconductive, making it valuable in producing superconducting magnets and electronics.
The Electron Configuration Of Niobium
Niobium’s electron configuration reveals that it has 41 electrons arranged in shells and subshells. This configuration plays a crucial role in determining its chemical and physical properties.
What Is Electron Configuration?
Electron configuration is the arrangement of electrons in an atom, which is fundamental to understanding its chemical and physical properties. It provides valuable insights into an element’s reactivity, stability, and bonding behavior. By knowing an atom’s electron configuration, scientists can predict how it will interact with other atoms and form compounds.
How Is Electron Configuration Represented?
Electron configuration is typically represented using the format 1s2 2s2 2p6 and so on, where the numbers and letters denote the different orbitals and the superscript numbers indicate the number of electrons in each orbital. The electron configuration is based on a set of rules known as the Aufbau principle, which states that electrons fill the lowest energy level orbitals first before moving to higher energy levels.
To further illustrate this, let’s take a closer look at the electron configuration of niobium.
Niobium Electron Configuration
Niobium, with the atomic number 41, is a transition metal with an interesting electron configuration. It can be represented as [Kr] 5s2 4d3 or in a more detailed format: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d3.
This configuration tells us that niobium has 41 electrons in total. It follows the Aufbau principle and starts with the filling of the 1s orbital, then the filling of the 2s and 2p orbitals, and so on. Niobium’s outermost electrons are found in the 5s orbital, with three of them occupying the 4d orbital. This arrangement contributes to niobium’s unique chemical and physical characteristics.
In conclusion, understanding the electron configuration of niobium gives us valuable insights into its properties and behavior. By studying the electron configurations of different elements, scientists can better comprehend the periodic table and the trends observed within it.
Understanding Niobium’s Electronic Structure
When it comes to understanding the properties and behavior of elements, a crucial aspect to consider is their electronic structure. In the case of niobium (Nb), a transition metal with atomic number 41, the arrangement of its electrons plays a significant role in its chemical and physical properties.
Valence Electrons
Valence electrons refer to the electrons present in the outermost energy level of an atom. They are involved in chemical bonding and determine an element’s reactivity. For niobium, with an atomic number of 41, it has a total of 41 electrons, and its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d4 5p1, revealing that niobium has 1 valence electron in its outermost 5p subshell.
Orbital Arrangement
Niobium, like other transition metals, has a complex electron orbital arrangement. Its electron configuration follows the Aufbau principle, which states that electrons first occupy the lowest energy orbitals before filling higher energy levels. In niobium, the electrons fill up the atomic orbitals in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, and 5p. This arrangement results in a stable electronic configuration for niobium.
Energy Levels
Energy levels in an atom refer to the different regions where electrons can be found. Each energy level is divided into sublevels or orbitals, accommodating a specific number of electrons. In niobium, the energy levels are designated by numbers, starting from 1 and increasing with energy. Niobium’s electron configuration shows that it has electrons in energy levels 1 to 5, with the outermost electrons occupying the fifth energy level.
Furthermore, niobium’s 4d and 5p orbitals are crucial in its reactivity and bonding behavior, allowing it to form compounds with various other elements.
In conclusion, understanding the electronic structure of niobium, including its valence electrons, orbital arrangement, and energy levels, provides insight into its chemical properties and reaction behavior. By examining these aspects, scientists can further explore and utilize niobium’s unique characteristics in different applications.
Significance Of Niobium’s Electron Configuration
Niobium’s electron configuration plays a significant role in determining its chemical reactivity, bonding characteristics, and magnetic properties. Understanding these aspects is crucial for various industrial applications and scientific research. Let’s delve into the significance of niobium’s electron configuration in these key areas.
Chemical Reactivity
Niobium’s electron configuration affects its chemical reactivity, determining how it interacts with other elements and compounds. The outermost electron configuration of 5s14d4 results in stable oxidation states, influencing its ability to form various chemical compounds.
Bonding Characteristics
The electron configuration of niobium influences its bonding characteristics, particularly in forming metallic bonds. With a partially filled d-subshell, niobium can readily participate in metallic bonding, contributing to its use in alloys and superconductors.
Magnetic Properties
Niobium’s electron configuration contributes to its magnetic properties. The presence of unpaired electrons in the d-subshell affects its magnetic behavior, making it significant in developing magnetic materials and technologies.
Factors Affecting The Electron Configuration Of Niobium
The electron configuration of niobium is determined by a few key factors that influence how its electrons are arranged. Understanding these factors is crucial to comprehend the behavior and properties of this element.
Atomic Number
The atomic number of niobium, which is 41, plays a significant role in determining its electron configuration. The atomic number indicates the number of protons in an atom’s nucleus, and in a balanced atom, it also equals the number of electrons. This means that niobium has a total of 41 electrons to distribute among its orbitals.
Orbital Occupancy Rules
The electron configuration of niobium follows certain rules based on the Aufbau principle, Pauli exclusion principle, and Hund’s rule. These rules dictate how electrons fill different atomic orbitals, ensuring that the energy levels are filled in a specific order.
| Orbital Type | Maximum Electron Capacity |
|---|---|
| 1s | 2 |
| 2s | 2 |
| 2p | 6 |
| 3s | 2 |
| 3p | 6 |
| 4s | 2 |
| 3d | 10 |
| 4p | 6 |
| 5s | 2 |
| 4d | 4 |
| 5p | 6 |
| 6s | 1 |
| 4f | 14 |
| 5d | 5 |
| 6p | 6 |
| 7s | 2 |
The table above shows the maximum electron capacity for different orbital types. By following these occupancy rules, we can determine how the 41 electrons of niobium distribute among its orbitals.
Comparison With Other Elements
When comparing niobium’s electron configuration with that of other elements, we can identify similar elements with different electron configurations and observe periodic trends. Understanding these comparisons allows us to gain further insights into niobium’s properties and behaviors.
Similar Elements With Different Electron Configurations
A notable element similar to niobium with a different electron configuration is tantalum (Ta), which is found directly below niobium in the periodic table. Both niobium and tantalum belong to Group 5 (VB) of the transition metals, characterized by their similar chemical properties.
However, the electron configuration of tantalum differs from niobium due to adding one more electron. While the electron configuration of niobium is [Kr] 5s2 4d4, tantalum has an additional electron occupying the 5d orbital, resulting in an electron configuration of [Kr] 5s2 4d4 5p6 5d3.
This difference in electron configuration between niobium and tantalum leads to variations in their physical and chemical properties, such as melting points, boiling points, and reactivity with other elements.
Periodic Trends
Examining the electron configurations of elements over a period allows us to observe certain periodic trends. Niobium is located in Period 5 of the periodic table, along with other elements like vanadium (V) and chromium (Cr).
One significant trend is the filling of the 3D orbital. Niobium has a half-filled 3D orbital, which contributes to its stability. Conversely, Vanadium has only one electron in the 3D orbital, while chromium possesses a completely filled 3D orbital.
This trend in the filling of the 3d orbital affects various properties, including magnetic behavior, as seen in the magnetic moment and paramagnetism of these elements.
To summarize, understanding the comparisons between niobium’s electron configuration and that of similar elements, as well as identifying periodic trends, helps unveil niobium’s unique characteristics and behavior within the periodic table.
Experimental Determination Of Niobium’s Electron Configuration
The experimental determination of niobium’s electron configuration is crucial to understanding its chemical properties and behavior. Spectroscopic techniques and quantum mechanical calculations play a significant role in uncovering this configuration.
Spectroscopic Techniques
Spectroscopic techniques, including X-ray photoelectron spectroscopy and ultraviolet photoelectron spectroscopy, are utilized to analyze the electronic structure of niobium. These methods use electromagnetic radiation to probe the energy levels and transitions within niobium’s atomic structure, providing valuable insights into its electron configuration.
Quantum Mechanical Calculations
Quantum mechanical calculations, such as Hartree-Fock and density functional theory, are employed to determine the distribution of electrons within niobium’s orbitals. These computational techniques allow for the prediction of the electron configuration through complex mathematical modeling, contributing to a deeper understanding of niobium’s electronic behavior.
Niobium’s Electron Configuration And Its Applications
Niobium, with an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1, boasts applications in various industries. Its prime utilization lies in creating superalloys for aerospace and medical devices. This versatile element also finds application in superconducting magnets and nuclear reactors.
Niobium is a chemical element with the symbol Nb and atomic number 41. Its electron configuration is crucial in defining its unique properties and applications in various fields. Let’s explore how the electron configuration of niobium contributes to its remarkable attributes.
Superconductivity
Niobium exhibits exceptional superconducting properties due to its electron configuration. Superconductivity refers to the phenomenon where certain materials possess zero electrical resistance when they are cooled below a critical temperature. In the case of niobium, its electron configuration allows the formation of Cooper pairs, resulting in the expulsion of magnetic fields and the absence of resistance in the flow of electrical current. This property makes niobium widely used in superconducting magnets, MRI machines, particle accelerators, and other applications where low-temperature superconductivity is essential.
Optical Properties
The electron configuration of niobium also influences its optical properties. Niobium has a unique ability to absorb and reflect specific wavelengths of light, making it useful in various optical applications. Its electron configuration determines the energy levels at which electrons can absorb photons and transition to higher energy states. This characteristic allows niobium to be utilized in developing high-performance coatings for lenses, mirrors, and other optical components. These coatings enhance the efficiency and precision of optical systems employed in research, telecommunications, and astronomy.
Catalytic Properties
Niobium’s electron configuration lends itself to remarkable catalytic properties. Catalysts are substances that speed up chemical reactions without being consumed in the process. Niobium-based catalysts are employed in various industrial applications, including petroleum refining, chemical synthesis, and environmental remediation. The electron configuration of niobium facilitates efficient electron transfer between reactants, enabling faster reaction rates and improved selectivity. By benefiting from niobium’s catalytic properties, industries can optimize production processes, reduce energy consumption, and minimize waste generation.
In conclusion, niobium’s electron configuration has far-reaching implications across different fields. Its superconducting properties make it indispensable in advanced technology, while its optical and catalytic properties contribute to innovations in optics and chemical processes. Understanding and harnessing niobium’s electron configuration allows us to explore its diverse applications and unlock its full potential in creating a sustainable and technologically advanced future.
Role Of Electron Configuration In Niobium Alloys
Electron configuration plays a crucial role in determining the properties of niobium alloys. By understanding the arrangement of electrons in niobium atoms, scientists can manipulate the alloy’s strength, corrosion resistance, and other desirable characteristics. The electron configuration of niobium allows for precise control over the alloy’s performance.
Strengthening Mechanisms
The role of electron configuration in niobium alloys is crucial for understanding their properties and behavior. Electron configuration refers to the arrangement of electrons within an element’s atomic orbitals, and it plays a significant role in determining how an element interacts with other atoms and compounds. In the case of niobium alloys, the electron configuration of niobium atoms affects their ability to incorporate other elements and form intermetallic compounds, influencing the alloy’s strength and performance.
Niobium alloys are known for their exceptional strength, making them valuable in various aerospace, automotive, and power generation industries. This strength is achieved through various strengthening mechanisms, all of which are influenced by electron configuration. One of the primary strengthening mechanisms in niobium alloys is solid solution strengthening, wherein the size and concentration of alloying elements affect the mobility of dislocations, making it more challenging for them to move and deform the material.
Another strengthening mechanism is precipitation hardening, where the formation of fine precipitates within the alloy matrix serves as obstacles to dislocation movement. The electron configuration of the alloying elements influences the types and sizes of precipitates that form and their distribution within the alloy, ultimately determining the extent of strengthening achieved. Different electron configurations can result in various types and distributions of precipitates, leading to distinct strengthening effects.
Intermetallic Compounds
The electron configuration of niobium atoms also plays a vital role in forming intermetallic compounds in niobium alloys. Intermetallic compounds are materials composed of two or more metallic elements, and they exhibit unique properties compared to pure metals or alloys. Intermetallic compounds can form in niobium alloys due to the interaction and bonding between niobium and other alloying elements.
The electron configuration of niobium atoms determines how readily niobium can react with other elements and form intermetallic compounds. The presence of specific electron orbitals in niobium’s outer shell can facilitate the bonding and stabilization of intermetallic compounds, leading to the creation of unique microstructures and mechanical properties in the alloy.
Understanding the role of electron configuration in intermetallic compound formation is crucial for designing and engineering niobium alloys with specific properties. By tailoring the electron configuration of niobium and alloying elements, researchers and engineers can manipulate the formation and behavior of intermetallic compounds, ultimately fine-tuning the strength, ductility, and other mechanical properties of niobium alloys for different applications.
In summary, electron configuration has a significant impact on the properties and behavior of niobium alloys by influencing strengthening mechanisms and intermetallic compound formation. By understanding how electron configuration affects these aspects, researchers and engineers can develop high-performance niobium alloys with tailored properties for various industrial applications.
Potential Future Developments In Niobium Electron Configuration
Potential Future Developments in Niobium Electron Configuration hold great promise for the advancement of various industries. They would allow for innovative applications and improved performance. Tailoring the electron configuration for specific applications and exploring emerging research areas are key components of this potential development.
Tailoring Electron Configuration For Specific Applications
Customizing the electron configuration of niobium opens up possibilities for enhancing its properties to meet the specific requirements of different industries. By deliberately adjusting the arrangement of electrons, scientists can optimize the material for applications in fields such as aerospace, electronics, and sustainable energy.
Emerging Research Areas
Niobium’s electron configuration continues to be at the forefront of research, with opportunities for further exploration in various applications. Researchers are delving into areas such as quantum computing, nanotechnology, and advanced materials, seeking to unlock the full potential of niobium’s electronic structure for cutting-edge advancements.
Challenges And Limitations In Studying Niobium’s Electron Configuration
Challenges and Limitations in Studying Niobium’s Electron Configuration
Studying the electron configuration of niobium presents several challenges and limitations. These obstacles arise due to experimental difficulties and the complexity of advanced theoretical modeling. Let’s explore these challenges in more detail.
Experimental Difficulties
When it comes to determining the electron configuration of niobium through experiments, researchers encounter various difficulties. Some of the key challenges include:
- Niobium’s high reactivity makes it challenging to prepare and stabilize pure samples for experimentation.
- Extreme conditions: Accurately studying niobium’s electron configuration requires precise experimental conditions, such as very low temperatures and high vacuum environments.
- Ionization potential: Niobium has a relatively high ionization potential, which means that it requires significant energy to remove an electron from its outer shell. This intricate process adds complexity to experimental measurements.
Advanced Theoretical Modeling
While experimental techniques provide valuable insights, advanced theoretical modeling is crucial in understanding niobium’s electron configuration. Some of the major considerations in this regard are:
- Quantum mechanical calculations: Sophisticated theoretical models based on quantum mechanics help predict the distribution and behavior of electrons in niobium’s orbitals. These calculations involve complex mathematical frameworks but offer crucial insights.
- Multipartite interactions: interactions between multiple particles, including electrons and nuclei, influence Niobium’s electron configuration. Accounting for these multipartite interactions adds further complexity to theoretical modeling.
- Supercomputer simulations: To accurately model niobium’s electron configuration, researchers often rely on powerful supercomputers to perform extensive simulations. These simulations help validate theoretical predictions and provide a deeper understanding of niobium’s electronic properties.
Considering the experimental difficulties and the intricacy of advanced theoretical modeling, unraveling niobium’s electron configuration requires a multidisciplinary approach. By combining experimental data with theoretical calculations, scientists can continue to expand our understanding of niobium and its electron behavior. Google Maps.
Frequently Asked Questions Of Niobium Electron Configuration
Why Is Niobium 4d4 5s1 Electron Configuration?
Niobium’s electron configuration is 4d4 5s1 because the 4d sublevel has a lower energy than the 5s sublevel.
What Is The Electron Configuration Of Nb 41?
The electron configuration of Nb 41 is [Kr] 5s2 4d3. It represents the arrangement of electrons in the energy levels.
Which Atom Has The Electron Configuration 1s22s2 2p6 3s2 3p4?
The atom with the electron configuration 1s22s2 2p6 3s2 3p4 is a sulfur atom (S).
What Element Has An Electron Configuration Of 1s2 2p6 2s2 3s2 3p5?
The element with an electron configuration of 1s2 2p6 2s2 3s2 3p5 is chlorine.
Conclusion
Understanding the niobium electron configuration is essential for grasping its chemical properties and behavior. It sheds light on the element’s role in various industries, including technology and manufacturing. By comprehending its electron arrangement, we gain insights into how niobium interacts with other elements and how it can be harnessed for practical applications.

