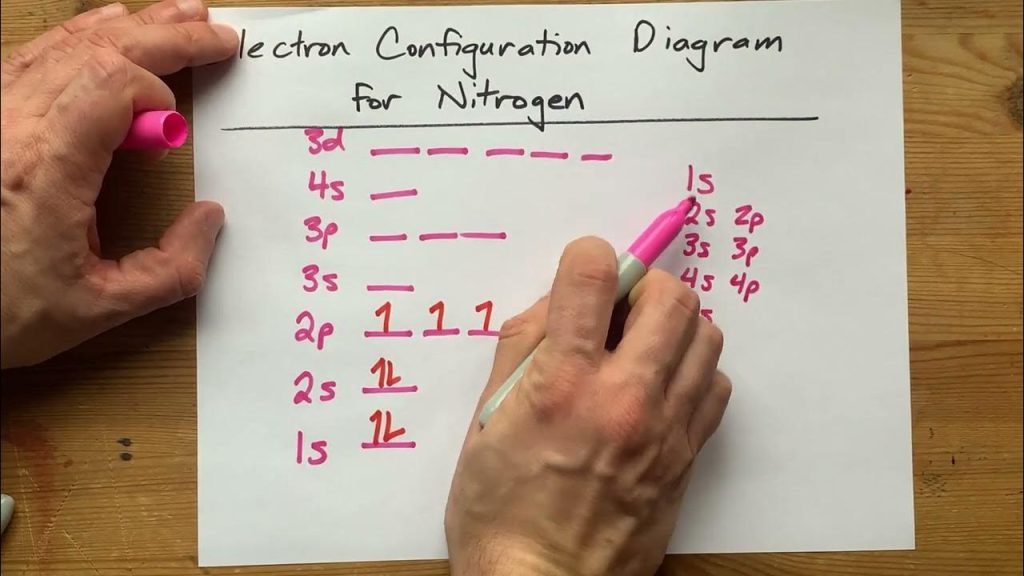
The electron configuration for nitrogen is 1s2 2s2 2p3, indicating that it has two electrons in its inner shell, two in the second, and three in the outermost shell. Nitrogen is a chemical element with the symbol N and atomic number 7.
Nitrogen is a colorless gas that forms about 78% of Earth’s atmosphere. It is essential for all plants and animals as it is an integral part of amino acids and proteins and a crucial component of DNA and RNA.
It is also used to produce ammonia, which is critical for making fertilizers, plastics, and explosives. Nitrogen has various isotopes and can form many different compounds with other elements, making it a versatile element with diverse applications in science and industry.
The Atomic Structure Of Nitrogen
Nitrogen has an electron configuration of 1s² 2s² 2p³, meaning it has a total of seven electrons in its atomic structure. The three electrons in its p orbital allow nitrogen to form various chemical bonds, making it an essential element in biological and industrial processes.
Nitrogen is a chemical element represented by the symbol ‘N’ in the periodic table. It is an essential component of all living organisms and an important element for industrial purposes. Nitrogen’s atomic number is ‘7,’ which means it contains seven protons and seven electrons. Nitrogen’s atomic structure includes an atomic nucleus, electrons, and energy levels. Understanding the atomic structure of nitrogen is essential to understanding its electron configuration, which plays a vital role in various chemical reactions. Let’s explore the subheadings related to nitrogen’s atomic structure.
The Number Of Electrons In Nitrogen
Nitrogen has seven electrons, which are distributed across different energy levels and sublevels. The first energy level contains two electrons, whereas the second energy level contains five electrons. Nitrogen’s outermost energy level contains three electrons, determining its chemical properties. These electrons participate in chemical bonding and make nitrogen a unique element.
The Energy Levels Of Nitrogen
Nitrogen’s electrons are positioned in different energy levels, representing an electron’s distance from the atomic nucleus. The first energy level, also known as the K shell, is closest to the atomic nucleus and contains two electrons. The second energy level, known as the L shell, is farther from the atomic nucleus and contains five electrons. Every energy level of nitrogen has sub-levels that determine the electron configuration. The number of energy levels and sublevels of nitrogen determines its electron configuration.
The Orbital Filling Order Of Nitrogen
The orbital filling order of nitrogen follows the Aufbau principle, which states that the electrons in an atom fill the lowest energy levels first before occupying higher energy levels. Nitrogen’s electrons occupy 1s, 2s, and 2p orbitals in the order of increasing energy levels. The 1s orbital contains two electrons, the 2s orbital contains two electrons, and the three 2p orbitals contain three electrons. The orbital filling order of nitrogen’s electrons is essential for understanding its electron configuration. In conclusion, nitrogen’s atomic structure determines its electron configuration, which is crucial in various chemical reactions. Understanding nitrogen’s electron configuration is essential for studying its chemical properties and reactions. Knowing the number of electrons, energy levels, and orbital filling order of nitrogen is crucial for understanding its electron configuration.
Electron Configuration For Nitrogen
Nitrogen is an element in the periodic table with an electronic configuration of 1s2 2s2 2p3. In other words, it has seven electrons in its outermost energy level and five electrons in its inner energy level. This post will discuss the notation used for electron configuration and the electron configuration of nitrogen in both the ground state and excited state.
The Notation Used For Electron Configuration
The notation used for electron configuration numerically describes where the electrons are located within an atom or molecule and their respective energy levels. This notation uses the principal quantum number, which is a positive integer, to denote the energy level. Letters s, p, d, and f indicate the subshell in which the electrons reside. The subshells s, p, d, and f correspond to l=0, l=1, l=2, and l=3, respectively.
The Electron Configuration Of Nitrogen In Ground State
The electronic configuration of nitrogen in the ground state is represented as 1s2 2s2 2p3, where the first number denotes the principal quantum number (n), and the letter indicates the subshell the electrons reside in (s or p). The superscript after each subshell letter represents the number of electrons present in that subshell. In this case, the element nitrogen has two electrons in the 1s subshell, two electrons in the 2s subshell, and three electrons in the 2p subshell.
The Electron Configuration Of Nitrogen In Excited State
Nitrogen, like any other element, can be excited to a higher energy level by adding energy. When nitrogen is excited, it goes from its ground state configuration of 1s2 2s2 2p3 to a configuration of 1s2 2s2 2p2 3s1, where there are two electrons in the 1s subshell, two electrons in the 2s subshell, two electrons in the 2p subshell, and one electron in the 3s subshell. This higher energy configuration is unstable, and the electron quickly returns to its original ground state configuration.
In conclusion, it is important to understand electron configuration for nitrogen and other elements as it helps us understand their chemical properties and how they interact with other elements in chemical reactions. The electronic configuration of nitrogen in both the ground and excited states can be determined using the notation presented above, which clearly represents the location and number of electrons within the atom.
Frequently Asked Questions For Electron Configuration For Nitrogen
How Do You Write The Electron Configuration For Nitrogen?
The electron configuration for nitrogen is 1s² 2s² 2p³.
What Is The Electron Configuration Of Nitrogen In 1s2 2s2 2p3?
The electron configuration of nitrogen in 1s2 2s2 2p3 has two electrons in the innermost shell, two in the second shell, and three in the third shell.
What Element Has The Electron Configuration 1s22s22p63s23p6?
The element with the electron configuration 1s22s22p63s23p6 is a noble gas called Xenon (Xe).
What Is The Electronic Configuration Of Nitrogen 1s2?
The electronic configuration of nitrogen: 1s2 2s2 2p3.
Conclusion
The electron configuration of nitrogen plays a crucial role in determining its chemical behavior. The periodic table and quantum mechanics help us understand how the electrons are distributed in the various energy levels.
By better understanding this configuration, we can also gain insights into how nitrogen behaves in different chemical reactions and how it interacts with other elements. Overall, having a solid understanding of electron configuration is essential for anyone studying or working in the field of chemistry.