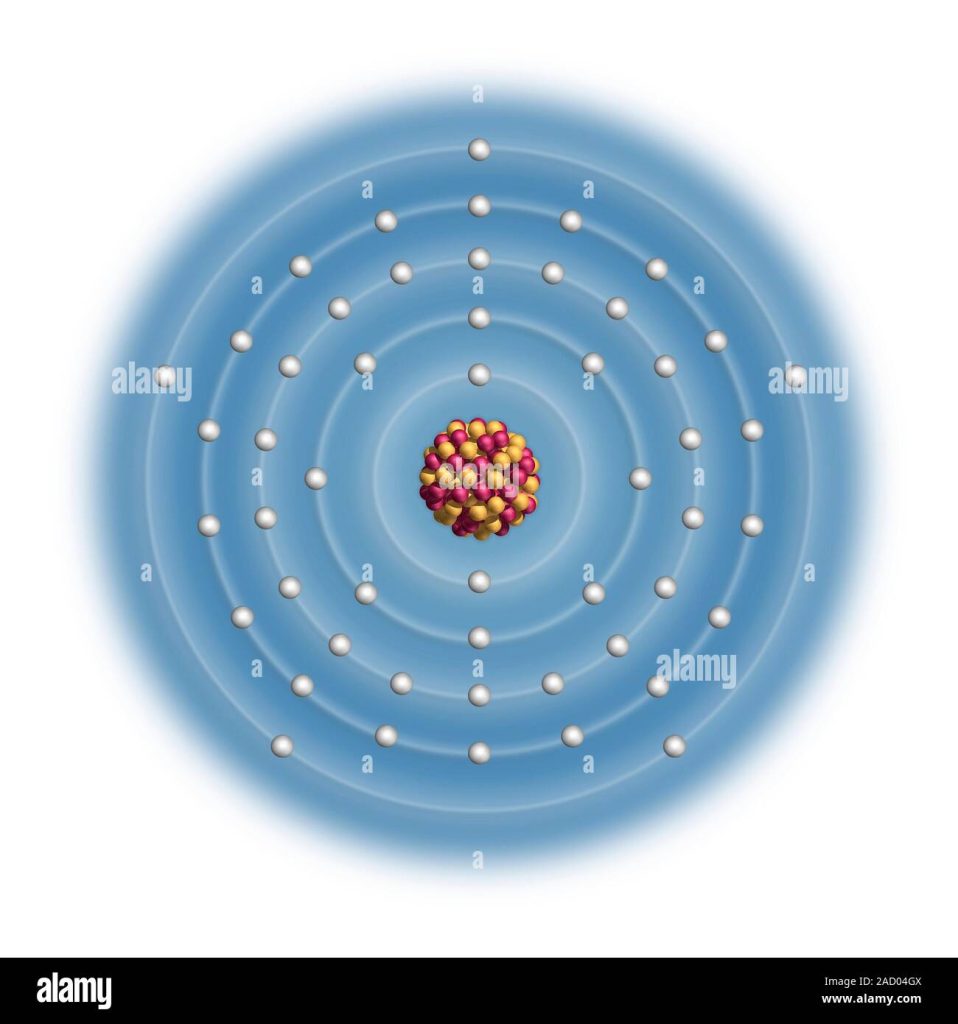
The electron configuration of Sb is 1s²2s²2p⁶3s²3p⁶3d¹⁰4s²4p⁶4d¹⁰5s²5p³. Antimony (Sb) has a valence electron configuration of 5s²5p³ and is a metallic element.
Antimony, a sulfide mineral typically found in nature, has properties similar to those of arsenic and other metalloids. It is used in a variety of applications, including as a flame retardant, in alloys with other metals, and in semiconductor production.
It can also be used to treat certain medical conditions, although it is toxic in high doses. We’ll explore the electron configuration of Sb and its applications in more detail.
Understanding Antimony
Antimony, a chemical element with the symbol Sb, has an electron configuration of [Kr] 4d10 5s2 5p3. This configuration indicates that antimony has five valence electrons in its outermost shell, making it a metalloid with properties of both metals and nonmetals.
Key Properties Of Antimony
Antimony is a naturally occurring chemical element and a metalloid that exhibits properties of both metals and nonmetals. Here are some key properties of antimony:
-
Melting point: 630.63K
-
Boiling point: 1587.15K
-
Density: 6.697 g/cm3
-
Atomic number: 51
-
Atomic symbol: Sb
-
Electron configuration: [Kr] 4d10 5s2 5p3
Antimony’s unique property of expanding as it cools from a molten state makes it useful in some applications, such as casting.
Applications Of Antimony
Due to its useful properties, antimony has a number of applications in various industries. Some notable applications of antimony are:
|
Industry |
Application |
|---|---|
|
Battery |
Antimony serves as a component in lead-acid batteries |
|
Fire Retardant |
Antimony trioxide is used as a fire retardant in plastics, textiles, and other materials |
|
Ceramics |
Antimony is used in the production of ceramics to improve their hardness and temperature resistance |
|
Medicine |
Antimony compounds have been used in the treatment of parasitic infections such as leishmaniasis |
|
Construction |
Antimony is an important component in the production of paint and glass |
In conclusion, understanding antimony and its key properties and applications is important in appreciating its significance in various industries. From batteries to medicine, antimony has proven to be useful and versatile in many applications.
Electron Configuration Basics
Antimony, also known as Sb, has an electron configuration of [Kr] 4d10 5s2 5p3. This configuration represents the arrangement of electrons in the various shells and subshells of the antimony atom. Understanding the electron configuration basics is crucial in understanding the behavior and reactivity of elements.
What Is Electron Configuration?
Electron configuration is the manner in which electrons are arranged in an atom’s orbitals. Orbital refers to the region where the electron is most likely to exist. Electron configuration describes the arrangement of the electrons in their respective orbitals for a given element.
Why Is Electron Configuration Important?
Electron configuration determines an atom’s chemical properties and how it will interact with other atoms. The number of electrons in an element’s outermost shell determines if it will lose, gain, or share electrons with other atoms. This interaction affects the element’s behavior and how it will react with other substances. It is essential to understand the electron configuration to understand the periodic table and its trends. The electron configuration contributes to the element’s place in the periodic table. The periodic table is divided into groups and periods based on the element’s valence electrons, which are the electrons in the outermost shell.
Electron Configuration Method
Two methods are used to represent the electron configuration: the orbital method and the Noble Gas method. The orbital method places each electron in its respective orbital. On the other hand, the Noble Gas method compresses the electron configuration by representing the outermost shell electrons in the same style as the previous Noble Gas element.
Example Of Electron Configuration
Let us take the example of Antimony. Antimony has 51 electrons, so its electron configuration is “1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3.” This configuration explains that the first shell has two electrons, the second shell has eight electrons, the third shell has 18 electrons, and the fourth shell has 15 electrons, with three electrons in the 4p subshell. In conclusion, understanding electron configuration is important to understand an element’s chemical and physical properties and how it will interact with other elements.
Sb Electron Configuration
The electron configuration of antimony (Sb) is a fascinating topic in chemistry. It describes the arrangement of electrons in an atom and determines many of its properties. Understanding Sb’s electron configuration is crucial for predicting its behavior in chemical reactions and understanding its chemical properties.
The electron configuration of Sb is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p3. This configuration can be broken down into different energy levels or electron shells, each containing a specific number of electrons.
Each electron shell is filled in a specific order, with the lowest energy level filling first. The Sb electron configuration follows the Aufbau principle, which states that electrons occupy the lowest available energy level before filling higher levels. Additionally, the Pauli exclusion principle mandates that each electron in an atom must have a unique set of quantum numbers. Finally, Hund’s rule states that the maximum number of unpaired electrons should be produced in a subshell when the electrons are distributed in different orbitals of a sub-shell of equal energy.
Following these principles, Sb gains stability by filling its outermost energy level with five valence electrons. This configuration makes Sb a metalloid, which exhibits properties of both metals and nonmetals.
Overall, the Sb electron configuration reveals important information about the atom’s properties and reactivity. It is a critical concept in the field of chemistry and helps us understand the behavior of this unique element.
Sb Electron Configuration Diagram
Antimony (Sb) has an electron configuration diagram that shows its 51 electrons in various energy levels and orbitals. The diagram can help chemists and students understand the properties and behavior of this element in chemical reactions and bonding.
The Sb Electron Configuration Diagram is an essential tool for understanding the electron arrangement in antimony (Sb). This diagram can be represented in several ways, but the most standard method is using orbital notations.
Standard Diagram Representation
The Sb Electron Configuration Diagram follows the Aufbau principle, which states that electrons fill orbitals starting from the lowest energy level to the highest. The diagram can be represented through a series of subshells, each holding a specific number of electrons. One way to represent the Sb Electron Configuration Diagram is through the use of superscripts and subscripts. The superscripts indicate the number of electrons found in each subshell, while the subscripts denote the energy level or shell in which the subshell is found. For example, the Sb electron configuration diagram can be represented as 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 5s^2 4d^10 5p^3
Understanding The Diagram
Understanding the Sb Electron Configuration Diagram requires a basic understanding of subshells and how electrons fill them. The diagram itself represents the arrangement of electrons in the different subshells of an antimony atom. Subshells are designated by letters, with s, p, d, and f representing different electron orbitals. The s subshell can hold two electrons, p can hold up to six, d can hold up to ten, and f can hold up to fourteen electrons. The number of subshells in a specific shell is equal to the shell number, and each subshell must be at least partially filled before electrons can fill the next subshell in that shell. By understanding these basic principles, you can easily interpret the Sb Electron Configuration Diagram and determine the electron arrangement of antimony.
Exceptions To Sb Electron Configuration
Antimony, with the atomic number 51, has an electron configuration of [Kr] 4d10 5s2 5p3. However, there are certain circumstances where antimony atoms deviate from this configuration. These deviations are known as exceptions to Sb electron configuration and generally occur due to stability reasons. In this section, we will discuss what these exceptions are and how they occur.
What Are Exceptions To Sb Electron Configuration?
Exceptions to electron configurations occur when the energy required to remove an electron from a subshell exceeds the paired electrons’ repulsion. Due to this phenomenon, atoms tend to achieve half-filled and fully-filled subshells in order to achieve stability. The exception to Sb electron configuration occurs when the 5s2 electron pair shifts to 4d10 subshell resulting in a half-filled 5s subshell and a fully-filled 4d subshell.
Explanation Of Exceptions
The Sb electron configuration exception makes the element more stable than it would be if it followed the typical electron configuration. When antimony is in its +3 oxidation state, it has a [Kr] 4d10 5s2 5p2 configuration, following Hund’s rule. However, in the +5 oxidation state, the electron configuration becomes [Kr] 4d10 5s2 4p3, which means that the 5s2 electrons have shifted to the 4p3 subshell.
The exceptional electron configuration of antimony shows that electron configurations are not always straightforward and predictable. Antimony is not the only element that exhibits this behavior; other elements also have exceptions to their electron configurations.
Summary
-
Exceptions to Sb electron configuration occur when the energy required to remove an electron from a subshell exceeds the paired electrons’ repulsion.
-
The exception to the Sb electron configuration occurs when the 5s2 electron pair shifts to the 4d10 subshell.
-
Antimony’s exceptional electron configuration enhances its stability.
Understanding Sb electron configuration exceptions can help predict the chemical behavior of antimony. Therefore, it is important to understand the exceptions and their implications.
Importance Of Sb Electron Configuration
The electron configuration of an element is critical in understanding its properties and behavior. Antimony, with the chemical symbol Sb, has a unique electron configuration that makes it important in different fields such as chemistry, physics, and materials science. In this blog, we will explore the relevance of Sb electron configuration in these different fields.
Relevance In Chemistry And Physics
Sb, positioned within group 15 of the periodic table, has an electron configuration of 1s22s22p63s23p63d104s24p64d105s25p3. This configuration allows for unique chemical binding properties that make antimony useful in creating chemical compounds such as trihalides.
Moreover, the half-filled 5s and 5p orbitals of antimony give it metallic properties that have applications in physics and electronic engineering. The very same electron configuration also allows antimony to exist as both a metal and a non-metal, making it an essential element that defies common classification.
Implications In Materials Science
The diverse properties of antimony make it a valuable component in a wide range of materials used in industrial and everyday products. For instance, antimony is popularly used as a flame retardant in textiles, plastics, and electronics due to its low flammability, which results from its unique electron configuration. Antimony trioxide, for example, prevents the spread of flames by dehydrating the nearby surfaces and forming a protective barrier.
Antimony is also commonly used to manufacture lead-acid batteries, as its high melting point and low vapor pressure make it useful in producing lead alloys. In addition, the element is a critical component in semiconductors used in infrared detectors, and its unique properties enable it to be used in infrared lenses, enabling the creation of sharp and clear images.
|
Application |
Material containing Antimony |
|---|---|
|
Flame Retardant |
Textiles, Plastics, Electronics |
|
Lead Acid Batteries |
Lead Alloys |
|
Semiconductors |
Infrared Detectors, Infrared Lenses |
Overall, antimony’s unique electron configuration has significant implications in various applications in chemistry, physics, and engineering, making it one of the world’s most valuable elements.
Frequently Asked Questions Of Sb Electron Configuration
What Is The Electronic Configuration Of Sb?
The electronic configuration of Sb is [Kr] 4d10 5s2 5p3, wherein Kr represents krypton, 4d10 and 5s2 represent the electron configuration of the inner noble gas core, and 5p3 represents the valence electrons in the outermost shell.
What Is Sb Configuration?
Sb configuration refers to the configuration of small business routers. It involves setting up the proper network settings to optimize internet connection and security features for business use.
What Is The Electron Configuration Of B?
The electron configuration of B is 1s² 2s² 2p¹.
How Many Electrons Are In The Element Sb?
There are 51 electrons in the element Sb.
Conclusion
The electron configuration of an element provides vital information on its chemical behavior and properties. It helps scientists predict the reactivity, stability, and bonding characteristics of atoms. Understanding the electron configuration of an element is fundamental to understanding its behavior in chemical reactions, as well as its applications in various fields, including medicine, technology, and industry.
Therefore, it is essential to study and comprehend electron configurations, as they are a cornerstone of modern chemistry. Keep exploring, and who knows what exciting discoveries await?