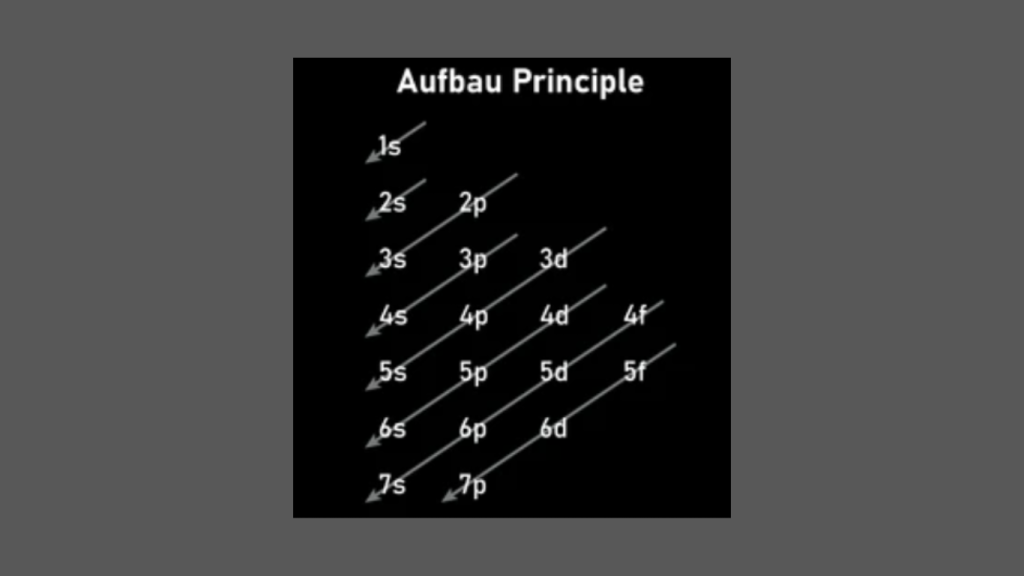
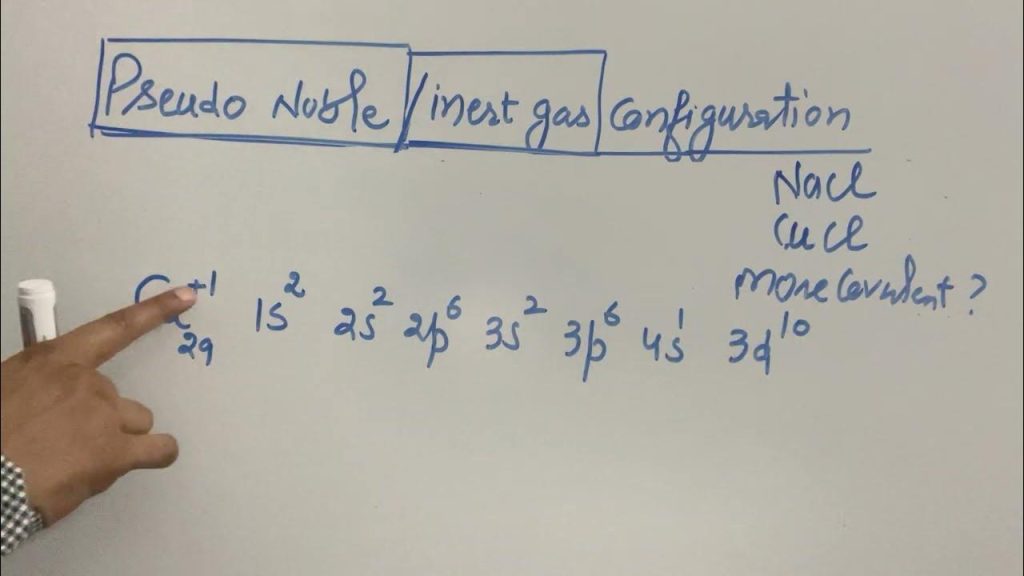Which Electron Configuration is That of an Element in the Fourth Period
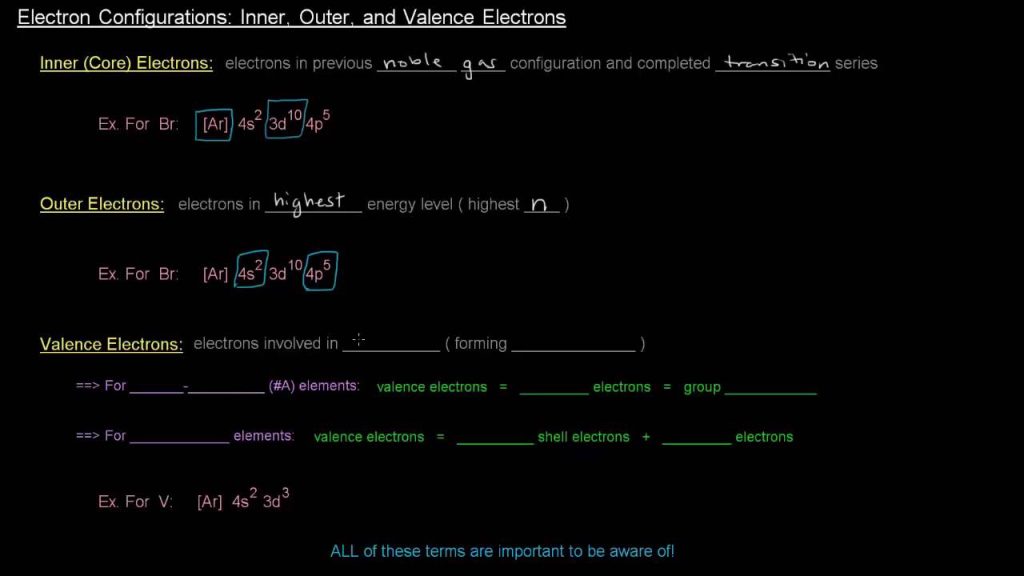
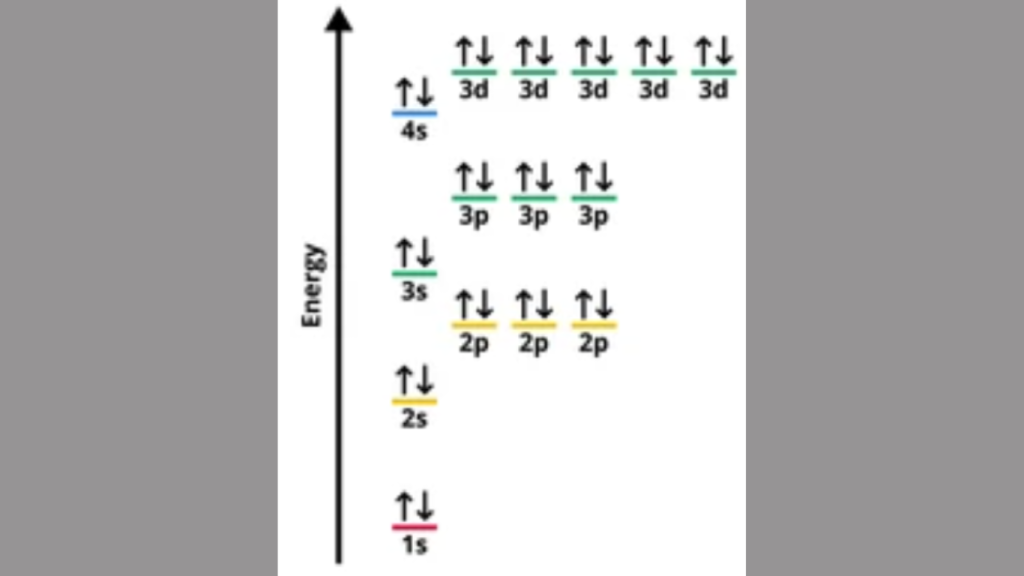
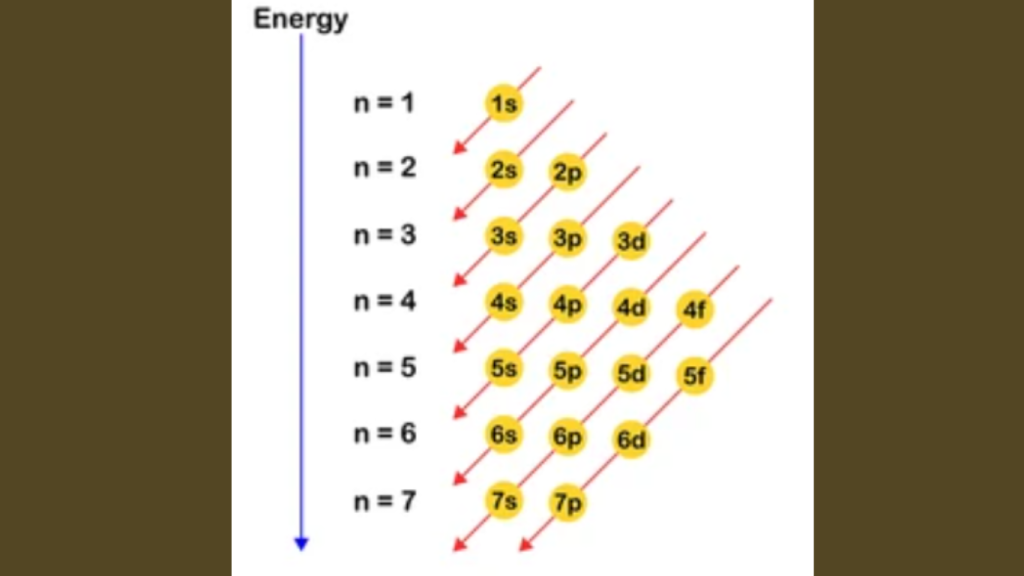
The electron configuration of an element in the fourth period is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6. The fourth period in the periodic table includes elements with electron configurations that follow the pattern of filling the 4s and 3d orbitals. Due to the overlapping of these orbitals, these elements exhibit diverse chemical properties, […]
Which Electron Configuration is That of an Element in the Fourth Period Read More »