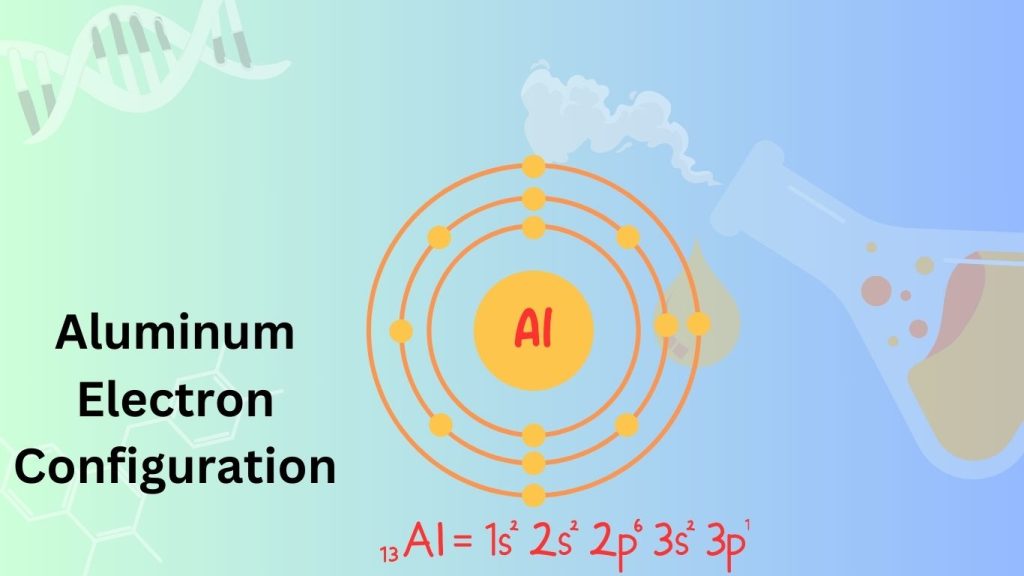
The Aluminum electron configuration of 1s^2 2s^2 2p^6 3s^2 3p^1. Aluminum is a chemical element that belongs to group 13 in the periodic table.
It is a lightweight and malleable metal known for its excellent conductivity and corrosion resistance. Aluminum is widely used in various industries, including construction, packaging, transportation, and electronics. Its electron configuration indicates that it has 13 electrons in total, with two in the first energy level, eight in the second energy level, and three in the third energy level.
This configuration allows aluminum to form stable compounds and participate in various chemical reactions.

What Is Aluminum?
Aluminum is a commonly used metal known for its strength, low density, and corrosion resistance. It is widely used in various industries, including aerospace, construction, and packaging.
Atomic Number And Symbol
Aluminum’s atomic number is 13, and its chemical symbol is Al. It belongs to the post-transition metals group.
Physical Properties
- Density: 2.70 g/cm³
- Melting Point: 660.32°C
- Boiling Point: 2519°C
- Color: Silvery-gray
Electron Configuration
Aluminum’s electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^1. It has 13 electrons distributed in its energy levels, making it a versatile element used in various industries.
Introduction
Understanding an atom’s electron configuration is a crucial aspect of studying chemistry. Electron configuration refers to the arrangement of electrons within an atom’s energy levels and orbitals, which determines an atom’s chemical properties and behavior. In this article, we will explore electron configuration and how it is determined.
What Is Electron Configuration?
Electron configuration is the distribution of electrons in an atom’s atomic orbitals. Electrons occupy different energy levels around the nucleus, which are represented by shells or principal quantum numbers. Each energy level consists of one or more atomic orbitals, which specify the probability of finding an electron in a particular region around the nucleus.
The electron configuration of an atom follows specific rules based on the Aufbau principle, Hund’s rule, and the Pauli exclusion principle. The Aufbau principle states that electrons first fill the lowest energy levels before moving to higher levels. Hund’s rule asserts that electrons will occupy separate orbitals with parallel spins before pairing up within a given energy level. The Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers, meaning each electron must have a unique combination of principal, azimuthal, and magnetic quantum numbers.
How Is Electron Configuration Determined?
Electron configuration can be determined using the periodic table and the knowledge of the aforementioned principles. The periodic table provides a valuable reference for identifying the number of electrons in each energy level and orbital. When determining an atom’s electron configuration, it is important to consider the element’s atomic number, which indicates the number of protons and electrons present.
To write the electron configuration in a condensed form, the following notations are often used:
- Noble Gas Notation: This notation utilizes the symbol of the noble gas located before the element in the periodic table to represent the configuration of the noble gas core (i.e., the completely filled inner electron levels). The remaining electron configuration is then written following the noble gas symbol.
- Orbital Notation: This notation represents each orbital, denoted by its energy level and the number of electrons present in that orbital. For example, 1s2 2s2 2p6 represents the electron configuration of neon (Ne), with two electrons in the 1s orbital, two in the 2s orbital, and six in the 2p orbital.
By understanding an atom’s electron configuration, scientists can gain insights into its reactivity, bonding properties, and possible chemical reactions. This fundamental concept lays the groundwork for various fields of chemistry, including chemical bonding, periodic trends, and spectroscopy.
The Electron Configuration Of Aluminum
Aluminum’s electron configuration is 1s2 2s2 2p6 3s2 3p1, with 13 electrons in total. This configuration signifies that there are 13 electrons surrounding a neutral aluminum atom. The arrangement of these electrons follows the rule that each orbital can hold a maximum of 2 electrons with opposite spins.
Aluminum is a versatile and widely used metal with various aerospace, construction, and electronics applications. Understanding its electron configuration is essential in comprehending its chemical properties and behavior. In this section, we will explore aluminum’s ground state and excited state electron configurations and delve into the concept of valence electrons.
Ground State Electron Configuration
The ground state electron configuration represents the arrangement of electrons in an atom’s lowest energy level. For aluminum, with an atomic number of 13, the ground state electron configuration can be represented as:
1s2 2s2 2p6 3s2 3p1
This configuration indicates that the first energy level (shell) contains two electrons in the 1s orbital, the second energy level has eight electrons distributed between the 2s and 2p orbitals, and finally, the third energy level holds three electrons, with two in the 3s orbital and one in the 3p orbital.
Excited State Electron Configuration
When an atom absorbs energy, some of its electrons can transition from their ground state to higher energy levels, leading to an excited state electron configuration. Let’s take a closer look at aluminum’s excited state configuration.
In an excited state, one of the 3s electrons of aluminum can be promoted to the vacant 3p orbital, resulting in the following excited state electron configuration:
1s2 2s2 2p6 3s1 3p2
This configuration highlights the movement of one electron from the 3s orbital to the 3p orbital. It is important to note that the excited-state electron configuration is temporary. As the electron transitions back to its ground state, it releases the absorbed energy in the form of light.
Valence Electrons
Valence electrons are found in the outermost energy level of an atom. These electrons are crucial in an element’s chemical properties and ability to form bonds with other elements. In the case of aluminum, the outermost energy level is the third energy level, containing three electrons in total.
Considering aluminum’s ground state electron configuration, which is 1s2 2s2 2p6 3s2 3p1, we can determine that it has only one valence electron located in the 3p orbital. This lone valence electron enables aluminum to readily form compounds through interactions with other elements.
Understanding aluminum’s electron configuration provides a foundation for comprehending its chemical reactivity and its involvement in a multitude of applications. By examining the ground state and excited state configurations and recognizing the significance of valence electrons, we gain insights into aluminum’s behavior in various chemical reactions and its role in different industries.
The Aufbau Principle
Understanding aluminum’s electron configuration is essential for comprehending its behavior in chemical reactions. At the core of this understanding lies the Aufbau Principle, a fundamental concept in chemistry that governs the order in which electrons occupy atomic orbitals.
Explanation Of Aufbau Principle
Electrons fill orbitals in an increasing order of energy levels. According to the Aufbau Principle, lower energy levels are filled before higher ones, and each orbital can hold a maximum of two electrons with opposite spins.
Application To Aluminum
Aluminum, with an atomic number of 13, has an electron configuration of 1s2 2s2 2p6 3s2 3p1. Following the Aufbau Principle, the electrons fill the 1s, 2s, 2p, and 3s orbitals sequentially until the 3p orbital, resulting in its stable configuration.
The Pauli Exclusion Principle
The Pauli Exclusion Principle, a fundamental concept in quantum physics, states that no two electrons in an atom can have the same set of quantum numbers. This means that only a maximum of two electrons with opposite spins can exist in a given orbital.
Explanation Of Pauli Exclusion Principle
The Pauli Exclusion Principle can be understood by considering the properties of electrons. Electrons, tiny particles orbiting the nucleus of an atom, possess certain quantum numbers that define their unique energy levels, orbital shapes, and orientations. According to the principle, each orbital can accommodate a maximum of two electrons.
Imagine electrons as guests at a party and orbitals as rooms available for them to occupy. The Pauli Exclusion Principle acts as the door policy, allowing only two guests with different spins (clockwise or counterclockwise) to occupy each room. This principle plays a crucial role in determining the chemical and physical behavior of elements, including Aluminum.
Application To Aluminum
Let’s explore how the Pauli Exclusion Principle applies specifically to the electron configuration of Aluminum:
1. Aluminum, with the atomic number 13, consists of a total of 13 electrons. These electrons are distributed across various energy levels and orbitals. Only the last three electrons determine its chemical properties.
2. Aluminum’s electron configuration can be represented as 1s² 2s² 2p⁶ 3s² 3p¹. This notation describes the distribution of electrons in specific orbitals, with the superscripts indicating the number of electrons present in each orbital.
3. This configuration shows that Aluminum has two electrons in the 1s orbital, two in the 2s orbital, six in the 2p orbital, two in the 3s orbital, and one in the 3p orbital.
4. Since the 2p orbital can accommodate a maximum of six electrons, the two remaining electrons in Aluminum’s electron configuration occupy separate 2p orbitals, each with an opposite spin.
In summary, the Pauli Exclusion Principle governs the distribution of electrons in atoms, ensuring stability and diverse chemical properties. Understanding this principle helps us comprehend the electron configuration of elements like Aluminum and enables scientists and researchers to explore the behavior of different elements in the periodic table.
Hund’s Rule
The Hund’s Rule governs the electron configuration of Aluminum, ensuring that electrons fill up orbitals singly before pairing up. It contributes to Aluminum’s unique properties and its position in the periodic table.
Explanation Of Hund’s Rule
Hund’s Rule is a fundamental principle in quantum mechanics that helps us understand how electrons are arranged within an atom. It states that when orbitals of equal energy are available, electrons will occupy different orbitals of the same energy before they start pairing up. In simpler terms, electrons prefer to occupy separate orbitals within the same energy level before sharing an orbital with another electron.
Application To Aluminum
Aluminum, with the chemical symbol Al, has an atomic number of 13, meaning it has 13 electrons. We can use Hund’s Rule and the periodic table to understand how these electrons are arranged. Starting from the first orbital, Aluminum’s electron configuration can be represented as 1s2 2s2 2p6 3s2 3p1.
Let’s break down the electron configuration of Aluminum and apply Hund’s Rule:
- The first two electrons occupy the 1s orbital, following the Aufbau Principle that electrons fill the lowest available energy levels first.
- Next, the 2s orbital is filled with two electrons, according to the Pauli Exclusion Principle, which states that each orbital can hold a maximum of two electrons with opposite spins.
- Now, we move to the 2p orbital, which can accommodate up to six electrons. Hund’s Rule tells us that the electrons will occupy the 2p orbitals separately, with their spins aligned in the same direction, before pairing up. So, Aluminum’s electron configuration continues with 2p6.
- Finally, the last electron fills the 3s orbital, as it is the next available orbital with the lowest energy level.
By understanding Hund’s Rule and applying it to Aluminum’s electron configuration, we can visualize how the electrons are distributed within the atom. This knowledge helps us understand aluminum’s chemical properties and behavior in various chemical reactions and bonding situations.
Why Is Aluminum’s Electron Configuration Stable?
Why is Aluminum’s Electron Configuration Stable?
Symmetry And Stability
Aluminum’s electron configuration stability can be attributed to its symmetry. Due to its 3 valence electrons and placement in the third energy level, it achieves stability through symmetry. The arrangement of electrons in its shells contributes to its stable configuration.
Energy Level Filling
Aluminum’s stable electron configuration is also related to energy level filling. With 13 electrons, aluminum fills its energy levels up to the 3p orbital, following the Aufbau principle, which helps maintain a stable configuration.
The Relationship Between Electron Configuration And Chemical Properties
Understanding the relationship between electron configuration and chemical properties is essential for comprehending the behavior of elements. The arrangement of electrons in an atom’s energy levels determines its reactivity with other elements and the formation of compounds. Let’s delve into these concepts further.
Reactivity With Other Elements
The electron configuration of an element plays a crucial role in determining its reactivity with other elements. Elements with incompletely filled outermost energy levels tend to be highly reactive as they strive to achieve a stable electron configuration. For example, metals like sodium (Na) have a single electron in their outer shell, making them extremely reactive. Metals tend to lose their outermost electrons to achieve a stable configuration, resulting in the formation of positively charged ions.
Formation Of Compounds
Compound formation involves combining elements to fill their outermost energy levels and achieve a stable electron configuration. This process is governed by the octet rule, which states that atoms tend to gain, lose, or share electrons to acquire a full set of eight electrons in their outermost energy level. By doing so, elements attain a stable configuration similar to the noble gases. For example, oxygen (O), with six valence electrons, readily bonds with two hydrogen (H) atoms, each contributing one electron, to form water (H2O).
Table: Examples of Elemental Reactivity Based on Electron Configuration
| Element | Electron Configuration | Reactivity |
|---|---|---|
| Lithium (Li) | 1s2 2s1 | Highly reactive |
| Fluorine (F) | 1s2 2s2 2p5 | Highly reactive |
| Neon (Ne) | 1s2 2s2 2p6 | Stable |
Overall, electron configuration plays a significant role in determining an element’s chemical properties. Understanding the relationship between electron configuration, reactivity, and compound formation allows us to comprehend the behavior of different elements and their interactions in chemical reactions.
The Role Of Electron Configuration In Aluminum’s Industrial Applications
Aluminum, with its unique electron configuration, plays a pivotal role in various industrial applications. Its electron configuration dictates its favorable properties, making it a preferred material in the construction and automotive industries. In this section, we will explore the significance of aluminum’s electron configuration in its industrial applications, focusing on its role as a structural material in electrical wiring and the automotive industry.
Aluminum As A Structural Material
Aluminum’s electron configuration contributes to its exceptional strength-to-weight ratio, making it the ideal choice for structural applications. With a light density of 2.7 g/cm³ and an atomic number of 13, aluminum possesses a stable electron configuration of [Ne] 3s2 3p1.
This configuration allows aluminum atoms to bond easily, forming a face-centered cubic (FCC) crystal structure. In this arrangement, the 3s electrons are involved in metallic bonding, while the 3p electron provides additional stability. The strong metallic bonds between atoms enable aluminum to withstand high loads while maintaining its structural integrity.
Aluminum In Electrical Wiring
The electron configuration of aluminum plays a crucial role in its application as an electrical conductor. With its valence electron in the 3p orbital, aluminum exhibits excellent electrical conductivity. Its electron configuration of [Ne] 3s2 3p1 allows the 3p electron to participate readily in the flow of electric current.
Furthermore, aluminum’s lower resistivity than many other metals makes it an efficient conductor. Its resistivity of only 2.65 x 10-8 Ω•m makes aluminum an attractive choice for electrical wiring, ensuring the efficient transmission of electricity.
Aluminum In The Automotive Industry
The electron configuration of aluminum influences its various advantages in automotive applications. Its [Ne] 3s2 3p1 configuration grants aluminum high corrosion resistance, allowing it to withstand harsh weather conditions and exposure to road salt. This property makes it an ideal material for the body panels of automobiles, extending their lifespan by reducing the risk of corrosion.
Additionally, aluminum’s lower density than steel enhances fuel efficiency and better vehicle performance. Its lighter weight, derived from its electron configuration, allows for increased fuel economy without sacrificing structural strength.
Overall, aluminum’s electron configuration is vital in its versatile industrial applications. Its unique properties, resulting from its specific electron arrangement, make aluminum an invaluable material, whether in structural applications, electrical wiring, or the automotive industry.
Aluminum’s Electron Configuration In The Periodic Table
Aluminum’s electron configuration in the periodic table is crucial in understanding its chemical properties and behavior. This configuration refers to the arrangement of its electrons within its energy levels and sublevels, which determines how it interacts with other elements. Let’s delve into the specifics of aluminum’s electron configuration and its placement in the periodic table.
Aluminum’s Group And Period
Aluminum belongs to Group 13 in the periodic table, also known as the boron group. It is located in Period 3, indicating that it has three energy levels in its electron configuration. The placement of aluminum in this group and period influences its atomic and chemical properties, such as its valence electrons and reactivity.
Comparison With Other Elements
Aluminum’s electron configuration stands out from other elements due to its unique arrangement of electrons in its orbitals. While it shares similarities with other elements in its group, such as boron and gallium, its electron configuration sets it apart, resulting in distinct properties and behaviors in chemical reactions.
Anomalies In Aluminum’s Electron Configuration
The electron configuration of an element provides valuable insight into its chemical properties and behavior. However, certain exceptions and anomalies arise when examining the electron configuration of aluminum. These anomalies can be explained by understanding the exceptions to the Aufbau principle and the concept of excited states in electron configurations.
Exceptions To The Aufbau Principle
The Aufbau principle states that electrons first fill the lowest energy levels before moving to higher ones. However, aluminum (symbol: Al) deviates from this principle in its electron configuration. Instead of having the expected electron configuration of 1s2 2s2 2p6 3s2 3p1, aluminum is found to have the electron configuration of 1s2 2s2 2p6 3s2 3p1.
This discrepancy is due to the stability of a fully or half-filled subshell. Aluminum can either fill its 3p orbital or move one electron from the 3s orbital to the 3p orbital. By moving the electron, aluminum can achieve a half-filled 3p subshell, which results in greater stability.
Excited States And Electron Configurations
Atoms can transition to higher energy states, known as excited states, by absorbing energy. This energy absorption allows electrons to move to higher energy levels, leading to standard electron configuration deviations. Aluminum can also exhibit excited states in its electron configuration.
For instance, aluminum’s ground state electron configuration is 1s2 2s2 2p6 3s2 3p1. However, when aluminum is excited, one of the electrons from the 3s orbital can be promoted to the 3d orbital, resulting in the excited state electron configuration of 1s2 2s2 2p6 3s1 3p1 3d1.
These excited states and deviations in electron configurations play a crucial role in aluminum’s chemical reactivity and properties. Understanding these anomalies can deepen our comprehension of aluminum’s behavior and its interaction with other elements. Google Maps.
Frequently Asked Questions About Aluminum Electron Configuration
How Do You Write The Electron Configuration For Aluminum?
The electron configuration for aluminum is 1s² 2s² 2p⁶ 3s² 3p¹.
What Is The Element With The Electron Configuration 1s22s22p63s23p5?
The element with the electron configuration 1s22s22p63s23p5 is chlorine.
What Is The Electron Configuration Of Al13?
The electron configuration of Al13 is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p1.
What Is The Electronic Configuration Of Al2+?
The electronic configuration of Al2+ is 1s2 2s2 2p6.
Conclusion
Understanding the electron configuration of aluminum is crucial in chemistry. It plays a vital role in its properties and behavior. By delving into the arrangement of its electrons, we gain insight into its stability and reactivity. This knowledge is essential in various scientific and industrial applications.
Mastering aluminum’s electron configuration opens doors to endless possibilities in materials science and engineering.