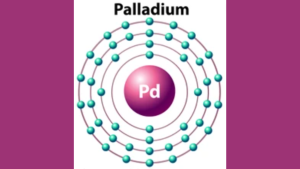
The Palladium Electron Configuration is [Kr] 4d10. Palladium has 46 electrons, with two in the s-orbital and ten in the d-orbital.
Palladium belongs to the transition metal group and is known for its exceptional catalytic activity, particularly in the automotive industry. Its electron configuration influences its chemical and physical properties, including its reactivity and ability to form coordination complexes. We will delve deeper into palladium’s electron configuration and its significance in understanding the element’s characteristics.
We will also explore some of its applications and uses in various fields, from industry to medicine.
What Is Palladium?
Palladium is a chemical element with the symbol Pd and atomic number 46. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10. It is a rare and lustrous silvery-white metal that belongs to the platinum group of elements.
Brief Introduction To Palladium
Palladium is a rare and lustrous silvery-white metal that belongs to the platinum group of elements. It was discovered in 1803 by William Hyde Wollaston, an English chemist and physician, and is named after the asteroid Pallas, which was discovered around the same time. Palladium’s scarcity, coupled with its unique properties, makes it highly sought after for use in a range of industries, including automobile manufacturing, jewelry, and electronics.
Physical And Chemical Properties Of Palladium
Palladium has the atomic number 46 and the symbol Pd on the periodic table. It is a transition metal with a melting point of 1,554.9 degrees Celsius and a boiling point of 2,963 degrees Celsius. Palladium is also highly ductile and malleable, which can be easily manipulated into different shapes and sizes. This metal is also known for its excellent chemical properties. For instance, it has a low reactivity to most chemicals, including oxygen and hydrochloric acid, making it very durable. Palladium is also one of the least dense metals, making it easy to work with and highly resistant to corrosion and tarnish.
Palladium Electron Configuration
Regarding electron configuration, palladium has six valence electrons distributed across its four energy levels. Its electron configuration is [Kr] 4d10, which means it has a completely filled 4d orbital and a half-filled 5s orbital. This unique electron configuration gives palladium its distinct characteristics, such as its ability to form coordination complexes and its catalytic activity. Palladium’s electron configuration also makes it a valuable component in producing catalytic converters for cars, which reduce harmful emissions by converting toxic gases into less harmful substances. In conclusion, palladium is a rare and valuable metal with unique physical and chemical properties. Its electron configuration plays a crucial role in its properties and makes it highly sought-after in various industries.
What Is Electron Configuration?
The electron configuration of palladium is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰. This configuration reflects the distribution of its 46 electrons across different energy levels and orbitals. Understanding an element’s electron configuration is fundamental for predicting its chemical and physical properties.
Electron configuration is the distribution of electrons among an atom’s energy levels, sublevels, and orbitals. It is crucial in determining its chemical and physical properties. An atom’s electron configuration depicts the number of electrons in each energy level and sublevel.
Explanation Of Electron Configuration
ls). The principal quantum number (n) indicates the energy level of the electron and can have whole number values from 1 to 7. The azimuthal quantum number (l) equals n – 1 and indicates the sublevel in which the electron is located. The magnetic quantum number (MLS) describes the spin of an electron around its axis. The electron configuration of an atom is written using the Aufbau principle, Hund’s rule, and the Pauli exclusion principle. The Aufbau principle states that electrons occupy the lowest energy level available first. Hund’s rule states that electrons fill each available sublevel singly, with an electron spin as parallel as possible until each sublevel contains one electron half-filled with opposite spins. Pauli’s exclusion principle states that no two electrons can have the same set of four quantum numbers.
Importance Of Electron Configuration In Chemistry
The electron configuration of an atom plays a significant role in determining an atom’s physical and chemical properties. It affects the atom’s reactivity, bonding behavior, and, in turn, the chemical properties of the elements. An atom’s electron configuration helps chemists understand why certain elements bond in specific ways. For example, noble gases have completely filled energy levels, and, as a result, they rarely react with other elements. In contrast, elements with incomplete outer energy levels are more reactive because they must gain, lose, or share electrons to attain a stable configuration. In conclusion, understanding electron configuration is fundamental in interpreting the behavior of elements in the periodic table. By studying the electron configuration, chemists can predict the properties and reactions of an element.
The Electron Configuration Of Palladium
The electron configuration of palladium is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d⁸. This transition metal has a total of 46 electrons, with the valence electrons being in the 4d shell.
Palladium is a rare and lustrous gray-white metal that is widely used in jewelry, electronics, and catalytic converters. Understanding the electron configuration of Palladium is crucial in many fields of science, including chemistry and physics. Palladium has 46 electrons arranged in four energy levels around the nucleus. In this post, we will explore Palladium’s ground state electron configuration and the explanation of valence electrons in Palladium.
Ground State Electron Configuration Of Palladium
The ground state electron configuration of Palladium is [Kr] 4d10 5s0. It indicates that the electrons in the Palladium atom are arranged in the lowest available energy levels. The [Kr] means that their inner shells shield the 36 electrons in Krypton from the valence electrons in Palladium. The 4d10 configuration represents ten electrons positioned in the d-orbital of the fourth energy level, while the 5s0 means no electrons are present in the 5s orbital of the fifth energy level.
Explanation Of Valence Electrons In Palladium
Valence electrons in Palladium refer to the outer-shell electrons, which participate in chemical bonding. Palladium has six valence electrons, two in the 4d-orbital and four in the 5s-orbital. The outermost valence shell of Palladium has a maximum capacity of 18 valence electrons, indicating that Palladium can bond with up to 18 elements. The electron configuration of Palladium plays a critical role in its chemical properties, particularly in its ability to form bonds with other elements. Palladium’s 5s and 4d electrons are responsible for its excellent catalytic properties and its ability to bond with hydrogen, making it an essential component of fuel cells. Additionally, these valence electrons also give Palladium its ability to form alloys with other metals, making it useful in jewelry manufacturing. Overall, the electron configuration of Palladium is a vital concept in understanding its properties and applications. This knowledge is essential in various fields, including material science, electronics, and catalysis.
Why Is Palladium’s Electron Configuration Important?
Palladium’s Electron Configuration is important because it determines the element’s properties and behavior. It affects its reactivity and interaction with other elements, making it crucial for industries like electronics and catalysis.
Palladium’s Electron Configuration And Its Properties
Palladium’s electron configuration is [Kr]4d^10. It has 46 electrons, with 28 electrons in its inner shell and 18 in its outer shell. Palladium is a transition metal that is a member of the platinum group. Palladium is known for its high melting point, ductility, malleability, strength, and resistance to tarnishing. Palladium is also used as a catalyst in various chemical reactions. One of the most interesting properties of palladium is its ability to absorb hydrogen gas. Palladium can absorb up to 900 times its own volume of hydrogen gas. This unique property is used in hydrogen storage and fuel cells.
Palladium’s Relationship To Other Elements In The Periodic Table
Palladium is located in Group 10 of the Periodic Table. It is in the same group as nickel and platinum. Palladium shares properties similar to these elements, such as a high melting point and resistance to corrosion. Palladium also has a similar electron configuration to other elements in Group 10. Palladium’s electron configuration allows it to form complex compounds with other elements. These compounds are used in various industrial applications, such as in synthetic rubber and plastics production. In conclusion, palladium’s electron configuration is important because it determines the element’s physical and chemical properties. Palladium’s ability to absorb hydrogen gas and its relationship to other elements in the Periodic Table contribute to its use in various industrial applications.
Applications Of Palladium
Palladium, a chemical element with the symbol Pd and atomic number 46, has a unique electron configuration that makes it useful in various applications. Its ability to absorb hydrogen and its catalytic properties contribute to its use in the automotive, electronics, and jewelry industries.
Palladium is a precious metal that has various applications in different fields. One of the most significant uses of palladium is in the manufacturing of catalytic converters for vehicles. The electron configuration of Palladium allows it to be effective in catalyzing reactions, which is why it is used in industries that require the conversion of one substance to another.
Industrial Uses Of Palladium
Palladium is widely used in the chemical and petroleum industries as a catalyst. It is employed in refining crude oil, which helps purify gasoline and other fuels. Palladium is also used in the production of semiconductors and electronic components, as well as in the manufacturing of jewelry and dental implants.
Palladium In Catalytic Converters And Its Role In Reducing Emissions
Catalytic converters in vehicles utilize the properties of palladium to reduce the harmful emissions that are emitted through the exhaust system. The palladium in the converter converts harmful gases such as carbon monoxide, nitrogen oxide, and hydrocarbons into less harmful gases like carbon dioxide, water vapor, and nitrogen gas. Using palladium in catalytic converters benefits the environment as it helps reduce air pollution. In recent years, stricter emission controls have been introduced all over the world, which has increased the demand for palladium used in catalytic converters. In conclusion, Palladium is a versatile precious metal with several applications. Its electron configuration makes it particularly useful in catalytic reactions and is thus widely employed in various industries. The use of palladium in the manufacturing of catalytic converters is an excellent example of the metal’s application to address a critical environmental issue.
Future Of Palladium
Palladium, a rare metal with a high electron configuration, is experiencing a rapid increase in demand due to its role in the automotive industry. As automakers shift towards cleaner-energy vehicles, the future of palladium looks promising, as it is a key component in catalytic converters that help reduce emissions.
Palladium is a rare silver-white metal widely used in various industries, including jewelry, electronics, and automobile manufacturing. The increasing demand for Palladium in these industries undoubtedly brings new opportunities to the metal market. As such, its future looks bright, given the metal’s unique properties that make it an irreplaceable element. In this article, we will examine the rising demand for Palladium, the challenges in its production, and its impact on industries.
Rising Demand For Palladium
In recent years, demand for Palladium has increased, driven largely by its critical role in the automotive industry. The metal’s unique properties make it essential for producing catalytic converters for gasoline-powered vehicles. With the increasing demand for cleaner, more efficient engines, the need for Palladium is also increasing. Additionally, Palladium’s exceptional conductivity makes it an ideal material for electrical applications such as electronics and telecommunications.
Challenges In Palladium Production And Its Impact On Industries
While Palladium’s demand is on the rise, the supply side of the metal is facing challenges. Palladium is a rare metal, and its primary source is from mines in Russia and South Africa. Political and economic instability in these countries often leads to supply disruptions, causing price spikes and market uncertainties. Moreover, the environmental impact of Palladium mining and production is significant. The mining process is energy-intensive, and greenhouse gas emissions from mining activities contribute to climate change. In addition, many of the metals’ primary applications, such as catalytic converters, rely heavily on using Platinum Group Metals (PGMs), which are expensive and complex to extract.
Here are some of the challenges in Palladium production:
| Challenges | Impact |
|---|---|
| Political instability in primary mining countries | Price spikes, market uncertainties |
| Environmental impact of mining and production | Contributing to climate change |
| Reliance on expensive and complex PGMs | Increasing production and operational costs |
To conclude,
the future of Palladium looks promising, given its unique properties and the increasing demand in different industries. However, it is essential to acknowledge and address the environmental and economic challenges in Palladium production. By exploring alternative sources and refining extraction techniques, the industry can leverage Palladium’s unique strengths while minimizing the environmental impact of its production.
Environmental Impact Of Palladium Mining
Palladium is a precious metal that is commonly used in the automotive and electronics industries. The demand for palladium has increased significantly over the years, leading to more palladium mining activities. However, palladium mining has some environmental impact, which affects the local communities and the environment. This article overviews the palladium mining process and the associated environmental concerns.
Overview Of The Palladium Mining Process
Palladium is mainly mined in two ways: underground mining and open-pit mining. Underground mining involves digging tunnels and shafts into the ground to access the palladium ore. On the other hand, open-pit mining involves removing the topsoil to expose the ore and then using heavy machinery to extract the palladium.
The palladium ore is then processed through a series of crushing and grinding before it is refined into pure palladium metal. The refining process involves the use of chemicals such as hydrochloric acid, nitric acid, and ammonia which generate waste products that are hazardous to the environment.
Environmental Concerns And Impact On Local Communities
Palladium mining activities have environmental concerns affecting the air, water, and soil. The main environmental concerns associated with palladium mining include:
- Water pollution—The chemicals used in the refining process can contaminate water sources, making them unsuitable for human consumption and harming aquatic life.
- Air pollution – Mining activities generate dust and emissions that can harm the air quality and affect the health of the local communities.
- Soil degradation—Open-pit mining involves removing the topsoil, leading to soil erosion, vegetation loss, and soil quality degradation.
- Waste disposal – The mining process generates waste products that contain hazardous chemicals, which need to be disposed of safely to avoid contaminating the environment.
The environmental impact of palladium mining is not limited to the environment alone. The noise, dust, and vibrations associated with mining activities can affect the health and well-being of the local communities. Some communities have reported respiratory problems, skin irritation, and hearing loss due to palladium mining.
Conclusion
Palladium mining is essential for meeting the demand for palladium in various industries. However, the environmental impact of palladium mining needs to be addressed to ensure sustainable mining practices. The mining companies need to implement measures to reduce the environmental impact of mining activities and protect the health and well-being of the local communities. Google maps
Frequently Asked Questions For Palladium Electron Configuration
Why Is Palladium Electron Configuration 4d10?
The 4d10 electron configuration of Palladium is due to the filling of its available energy levels. The 4d subshell has a lower energy level than the 5s subshell, causing the electrons to occupy the 4d subshell before filling the 5s subshell.
What Element Has An Electron Configuration Of 1s 2 2s 2 2p 6 3s 2 3p 4?
The element with an electron configuration of 1s2 2s2 2p6 3s2 3p4 is sulfur.
What Is The Electron Configuration Of Pd Ii?
The electron configuration of Pd II is [Kr] 4d8.
What Is The Full Electron Configuration For Platinum?
The full electron configuration of Platinum is [Xe] 4f^14 5d^9 6s^1.
Conclusion
The palladium electron configuration is an essential topic of study for chemists and physicists alike. Understanding the electronic structure of this unique metal can provide insights that help develop new materials and technologies. By exploring the various types of electron configurations possible for palladium, we can deepen our understanding of its behavior and properties.
If you’re interested in the fascinating world of chemistry and materials science, examine the electron configuration of palladium.
