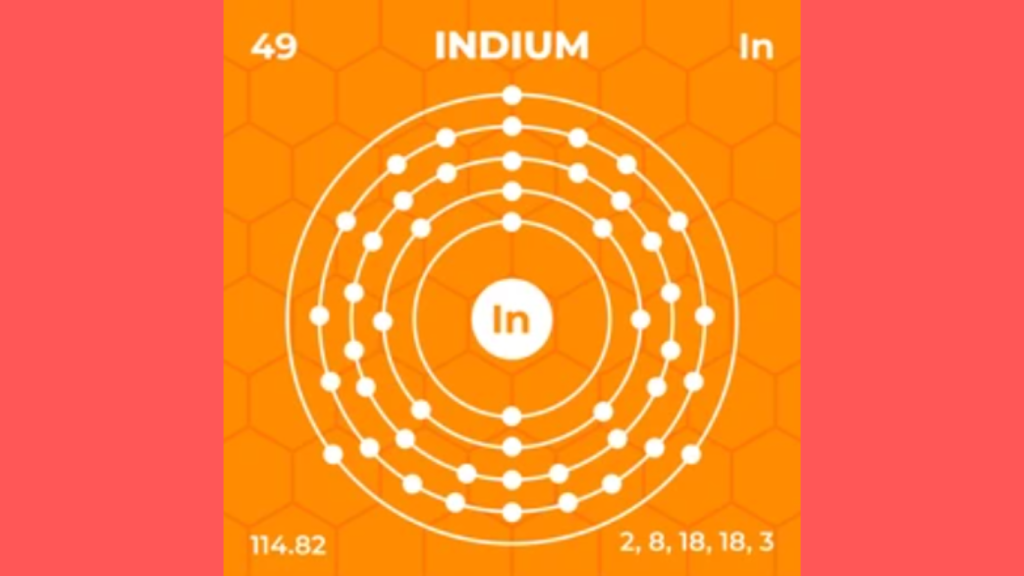
The electron configuration for Indium is [Kr] 4d10 5s2 5p1. Indium has 49 electrons distributed among its shells, with the outermost shell consisting of one electron in the p orbital.
Indium is a soft, silvery-white metal with a melting point of 156. 6°C and a boiling point of 2072°C. It is a relatively rare element, accounting for only 0. 025 parts per million of the Earth’s crust. Due to its unique properties, such as its low melting point and ability to conduct electricity, indium is widely used in the electronics industry.
It also has applications in areas such as nuclear medicine, thin film coatings, and solar cells. We will delve deeper into the electronic configuration of indium, its properties, and its uses.
Methods For Finding Electron Configuration
Indium is a chemical element with the symbol In and atomic number 49. The electron configuration for Indium is an important aspect of the component as it helps to understand its chemical properties. There are different methods for finding the electron configuration of Indium, namely the Aufbau principle, Hund’s rule, and Pauli exclusion principle. Let’s take a closer look at each method.
Aufbau Principle
The Aufbau principle is a German word meaning “building up.” It is used to determine the electron configuration of an element based on its atomic number and the energy levels of its electrons. The Aufbau principle states that electrons first fill the lowest energy orbitals before moving to higher energy orbitals. Therefore, the electron configuration for Indium can be determined by filling its electrons in the increasing order of energy levels. The electron configuration of Indium according to this principle is:
| Energy Level | No. of Electrons |
|---|---|
| 1s2 | 2 |
| 2s22p6 | 10 |
| 3s23p63d10 | 18 |
| 4s24p64d105s24f14 | 13 |
Hund’s Rule
Hund’s rule is another method used to determine the electron configuration of an element. It states that electrons will occupy empty orbitals before occupying orbitals with existing electrons. According to Hund’s rule, Indium’s electron configuration is:
- 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p1
Pauli Exclusion Principle
Lastly, the Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers. Therefore, the electron configuration for Indium, based on the Pauli exclusion principle, is as follows:
- 1s22s22p63s23p64s23d104p65s24d105p1
In conclusion, the electron configuration of Indium can be determined using different methods, such as the Aufbau principle, Hund’s rule, and the Pauli exclusion principle. Understanding an element’s electron configuration is crucial as it helps uncover its chemical properties.
Frequently Asked Questions On Electron Configuration For Indium
How Do You Write The Electron Configuration For Indium?
The electron configuration for indium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p1.
What Element Has An Electron Configuration Of 1s 2 2s 2 2p 6 3s 2 3p 4?
The element with an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 4 is sulfur (S).
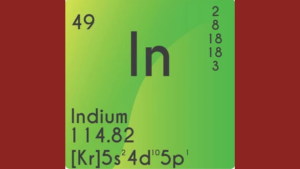
What Is The Electron Configuration Of 49 In?
The electron configuration of 49 In is 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p¹.
What Is The Electron Configuration Of 1s2 2s2 2p6 3s2 3p6 3d10 4s1?
The electron configuration of 1s2 2s2 2p6 3s2 3p6 3d10 4s1 is the arrangement of electrons in an atom or ion within its orbitals. In this configuration, there are 2 electrons in the first shell, 8 electrons in the second shell, 18 electrons in the third shell, and 1 electron in the fourth shell.
Conclusion
Understanding electron configuration is crucial in comprehending the behavior of atoms and their chemical reactions. This post has detailed the electron configuration of Indium and explained its properties and characteristics. With this knowledge, you can better identify elements and predict their chemical behavior.
Stay curious and keep learning!