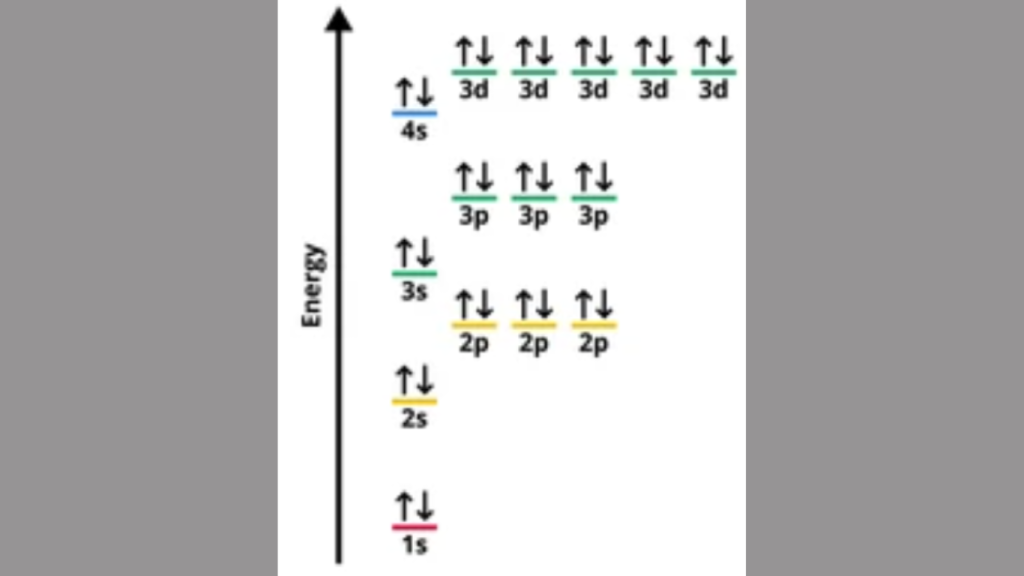
The significance of electron configuration lies in understanding the arrangement of electrons in an atom’s energy levels, which determines its chemical properties. Electron configuration plays a crucial role in explaining the reactivity, bonding, and stability of elements.
Electron configuration refers to the distribution of electrons in an atom’s orbitals, providing essential information about an element’s behavior in chemical reactions. It dictates an element’s ability to form compounds and participate in reactions, influencing its bonding and physical properties.
Understanding electron configuration helps predict an element’s behavior in various chemical reactions and understand the periodic trends in the periodic table. Because it is significant in determining an element’s chemical properties and behavior, electron configuration is a fundamental concept in chemistry, essential for comprehending the nature of matter and its interactions.
Importance Of Electron Configuration
The significance of electron configuration lies in its role as the basis of chemical properties. An element’s behavior is determined by its electron arrangement within its atoms. The way electrons are distributed in different energy levels, and orbitals greatly influence an element’s reactivity, bonding capabilities, and chemical reactions.
Electron configuration provides vital information about an element’s valence electrons, which are responsible for forming bonds with other atoms. These valence electrons determine an element’s tendency to gain, lose, or share electrons to achieve a stable configuration, thereby influencing its chemical behavior and reactions.
Understanding electron configuration allows scientists to predict an element’s properties and behavior, helping create new materials, drugs, and chemical compounds. It provides insights into the arrangement of electrons in atoms, enabling a deeper comprehension of the periodic table and its trends and patterns.
Fundamentals Of Electron Configuration
Understanding the significance of electron configuration is crucial in chemistry. Electrons are distributed in specific energy levels and orbitals based on quantum numbers, and this arrangement determines an element’s chemical properties.
Electron Configuration Patterns
Understanding electron configuration patterns is crucial in chemistry. The arrangement of electrons in an atom follows specific trends on the periodic table. Valence electrons, located in the outermost energy level of an atom, determine an atom’s reactivity and bonding behavior.
Role In Bonding And Reactivity
The electron configuration of an atom plays a crucial role in determining its bonding and reactivity. In terms of chemical bonding, the distribution of electrons in different energy levels and orbitals affects how atoms interact with one another. It determines whether atoms will form ionic, covalent, or metallic bonds. Electrons in the outermost energy level, known as the valence electrons, are particularly significant as they bond. The electron configuration of an atom also influences its reactivity. Elements with incomplete or highly stable electron configurations tend to be more reactive as they strive to achieve a more stable arrangement. The number and arrangement of valence electrons determine an element’s reactivity, such as its ability to gain, lose, or share electrons in chemical reactions.
Significance In Spectroscopy
Understanding electron configuration in spectroscopy is critical as it determines an element’s unique spectral lines. By examining electron arrangement, scientists can identify elements and their properties through spectral analysis, aiding various research fields.
| Significance in Spectroscopy |
| Interpreting Spectral Lines: Electron configuration is crucial for interpreting spectral lines. The arrangement of electrons in atoms influences the energy levels and transitions, leading to specific spectral lines. |
| Identifying Elements: By understanding electron configuration, scientists can identify elements based on their unique spectral fingerprints, aiding in various applications such as astronomy and chemistry. |
Implications In Material Science
Understanding the significance of electron configuration is crucial in material science. Electron configuration directly impacts the electronic properties of materials, determining their behavior in various engineering applications. Specifically, the arrangement of electrons in an atom’s orbitals dictates its conductivity, magnetism, and optical properties. This knowledge is invaluable in developing advanced electronics, energy storage, and catalysis materials. Additionally, electron configuration influences the reactivity and stability of compounds, guiding the design of new materials with tailored properties. In summary, a deep grasp of electron configuration is essential for harnessing the potential of materials in diverse technological and industrial applications.
Understanding Complex Electron Configurations
The Electron Configuration is of great significance as it helps us understand the arrangement of electrons in an atom. Complex electron configurations, especially those of transition metals and rare earth elements, are crucial for understanding their unique properties and reactivity. Transition metals, located in the d-block of the periodic table, possess partially filled d orbitals. This electron configuration allows them to exhibit various oxidation states and form colorful compounds. Rare earth elements in the f-block have electron configurations, with 14 electrons filling the 4f subshell. These elements have diverse applications in technology, such as magnets, lasers, and catalytic converters. By understanding the complex electron configurations of these elements, scientists can harness their properties for practical purposes and further expand our knowledge of the atomic world.
Techniques For Electron Configuration Determination
Understanding electron configuration is crucial in chemistry, as it determines an element’s properties. Various methods can be employed to determine electron configuration, such as quantum mechanical calculations and spectroscopic techniques. Quantum mechanics helps predict the arrangement of electrons around the nucleus. Spectroscopy, on the other hand, utilizes light absorption to analyze electron configurations. These techniques are essential for studying chemical reactions and understanding elemental behavior. Google maps
Conclusion
Understanding electron configuration is crucial for comprehending atoms’ behavior and properties. Scientists can predict chemical reactivity, bonding patterns, and even the color of certain elements by knowing the arrangement of electrons within an atom’s energy levels and orbitals.
It is a fundamental concept in chemistry, providing a foundation for various fields such as materials science, biochemistry, and quantum mechanics. Therefore, delving deeper into electron configuration can unlock a wealth of knowledge about the universe at the atomic level.