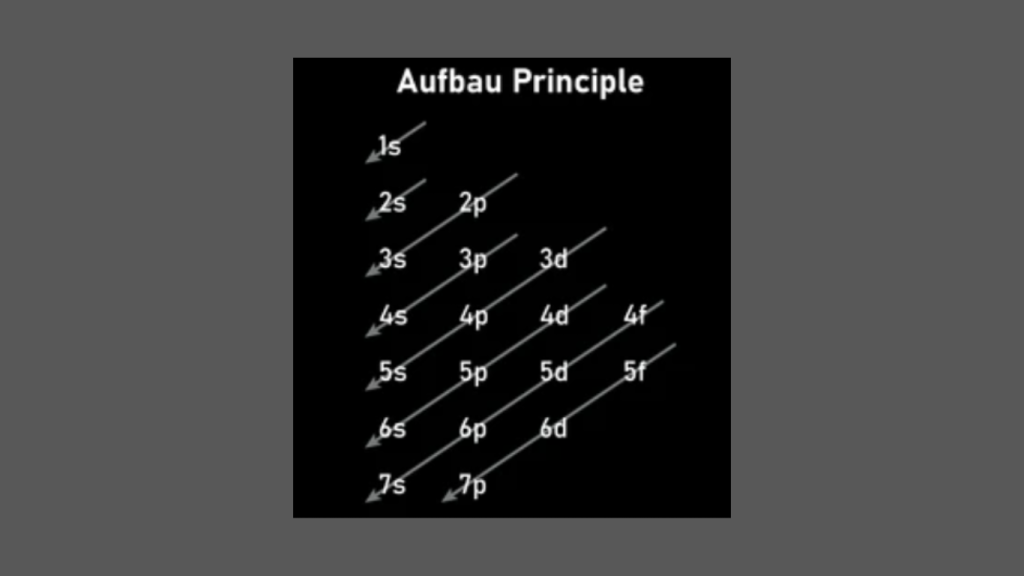
The Rule of Electron Configuration determines the distribution of electrons in an atom’s electron shells. This rule follows the order of filling the subshells with electrons.
Understanding the Rule of Electron Configuration is crucial in comprehending the behavior of atoms in chemical reactions and their bonding properties. Electron configuration is fundamental in predicting an element’s chemical reactivity and its properties. It provides insight into an element’s stability and its ability to bond with other elements to form compounds.
By following the rule of electron configuration, scientists and chemists can understand and predict the behavior of elements and their interactions with each other, contributing to advancements in various fields such as material science, pharmaceuticals, and environmental studies.
The Basics Of Electron Configuration
What are Electrons? Electrons are tiny particles that orbit the nucleus of an atom.
Atomic Structure – Atoms consist of protons, neutrons, and electrons.
What is Configuration? – Configuration refers to the arrangement of electrons in an atom.
Understanding Energy Levels – Electrons occupy different energy levels within an atom.
Orbitals and Subshells – Orbitals are regions in an atom where electrons are likely to be found.
The Rule Of Electron Configuration
The Rule of Electron Configuration dictates how electrons are arranged in an atom’s electron shells, following several principles for stability.
What Is The Rule?
The Rule states that electrons occupy the lowest energy levels first before moving to higher energy levels, creating electron configurations.
The Aufbau Principle
Electrons fill orbitals in order to increase energy levels, starting with the lowest energy orbit.
Hund’s Rule
Each orbital in a subshell is singly occupied with parallel spins before pairing up, maximizing electron separation.
Pauli Exclusion Principle
States that no two electrons in the same atom can have the same set of four quantum numbers, ensuring electron uniqueness.
Exceptions To The Rule
Exceptions to the Rule of Electron Configuration can occur when transitional metals defy typical patterns. These anomalies can occur when electrons fill subshells differently than expected, causing deviations in electron configuration predictions. Understanding these exceptions is crucial for accurately determining electron arrangements in elements.
Transition Metals
Transition metals are a unique group on the periodic table that exhibit exceptions to the rule of electron configuration. These elements have partially filled d orbitals, which can result in variations from the expected electron configuration patterns. Instead of filling orbitals in a strict numerical order, transition metals can have electrons distributed to maximize stability. This allows for forming compounds with different oxidation states, a significant characteristic of transition elements.
Half-filled And Fully-filled Orbitals
An interesting exception to the rule of electron configuration occurs when an orbital becomes either half-filled or fully filled. These configurations offer additional stability due to the way electrons interact with one another. When an orbital is half-filled, such as with elements in group 5 (VA) of the periodic table, the interaction between electrons is maximized, resulting in increased stability. Similarly, when an orbital is fully-filled, like with elements in group 8 (VIIIA) of the periodic table, the repulsion between electrons is minimized, leading to enhanced stability.
Quantum Mechanical Effects
The world of quantum mechanics brings another layer of complexity to the rule of electron configuration. Quantum mechanical effects, such as electron-electron interactions and the uncertainty principle, can influence how electrons are distributed in an atom. These effects can lead to variations in electron configuration, especially in atoms with higher atomic numbers. For example, the inert pair effect, observed in elements with electrons in the p orbitals, occurs when the outermost s electrons are not involved in bonding, leading to deviations from the expected electron configuration.
Applications Of Electron Configuration
Discover the significance of electron configuration rules in various applications across chemical bonding and spectroscopy. Understanding these rules helps predict element reactivity and behavior in different chemical reactions and environments. Proper electron configuration plays a crucial role in determining the properties and characteristics of atoms and molecules.
`chemical Properties And Reactivity`
`Understanding the electron configuration of an element is essential in predicting its chemical properties and reactivity. The arrangement of electrons in the electron shells of an atom directly influences its behavior in chemical reactions. By knowing the electron configuration, we can anticipate how an element will react with other elements and compounds, as well as its ability to form ions.`
`predicting Bonding And Molecular Structure`
`Electron configuration is crucial in predicting how atoms will bond and the resulting molecular structure. Knowing the distribution of electrons allows us to determine whether an atom will lose, gain, or share electrons to achieve a stable configuration. This understanding is fundamental in determining the type of bond that will form (ionic, covalent, or metallic) and the shape and properties of the resulting molecules.`
`understanding Periodicity`
`The concepts of electron configuration play a significant role in understanding periodic trends in the periodic table. By analyzing the electron configurations of elements, we can observe patterns in their properties such as atomic radius, ionization energy, and electronegativity. These trends help us make predictions about the behavior of elements within their respective groups and periods, providing valuable insights for various scientific applications.`
Experimental Determination Of Electron Configuration
The rule of electron configuration is a fundamental concept in chemistry that governs the arrangement of electrons in an atom. Experimental determination of electron configuration involves observing the energy levels and sublevels to determine the precise arrangement of electrons within an atom’s orbitals, providing valuable insights into the behavior and properties of elements.
Experimental determination of electron configuration refers to determining the arrangement of electrons within an atom’s orbitals through various spectroscopy techniques and X-ray crystallography.
Spectroscopy Techniques
Spectroscopy techniques analyze the interaction between matter and electromagnetic radiation. These include methods such as UV-visible spectroscopy, infrared spectroscopy, and photoelectron spectroscopy, which provide valuable insights into atoms’ energy levels and electron configurations.
X-ray Crystallography
X-ray crystallography is a powerful technique used to determine the arrangement of atoms within a crystal lattice. By analyzing the diffraction pattern produced by X-rays passing through a crystal, scientists can infer the electron distribution and configuration within the atoms, providing crucial information about the electron arrangement. By employing these experimental methods, researchers can accurately determine the electron configuration of various elements, contributing to our understanding of the fundamental properties of matter.
Practical Examples And Exercises
It’s important to go through practical examples and exercises to understand the concept of electron configuration better. Applying the rule of electron configuration to real scenarios makes it easier to grasp the core principles and enhances problem-solving skills. Let’s dive into some example calculations, explore the electron configurations of different elements, and engage in problem-solving exercises.
Example Calculations
Calculating electron configurations may seem overwhelming initially, but with practice, you’ll become more comfortable with the process. Let’s consider an example calculation to better comprehend the rule of electron configuration.
For instance, let’s determine the electron configuration of nitrogen (N), which has an atomic number of 7:
- First, we assign electrons to the available energy levels or shells in order of increasing energy. The available energy levels are represented by numbers: 1, 2, 3, and so on.
- Nitrogen’s atomic number indicates that it has seven electrons. We fill these electrons in the energy levels, starting from the lowest. The first two electrons go into the first energy level, the next three go into the second energy level, and the remaining two go into the third energy level.
- The electron configuration of nitrogen can be represented as 1s2 2s2 2p3.
Electron Configurations Of Elements
Understanding the electron configurations of different elements is essential in chemistry. The electron configuration determines an element’s chemical properties and behavior in chemical reactions. Let’s take a look at the electron configurations of a few elements:
| Element | Electron Configuration |
|---|---|
| Lithium (Li) | 1s2 2s1 |
| Oxygen (O) | 1s2 2s2 2p4 |
| Calcium (Ca) | 1s2 2s2 2p6 3s2 3p6 4s2 |
Problem-solving Exercises
To enhance your understanding and application of electron configuration, practicing problem-solving exercises is crucial. Let’s try solving the following exercise:
What is the electron configuration of chlorine (Cl), which has an atomic number of 17?
- Assign electrons to the energy levels, starting from the lowest. Fill the first two electrons in the first energy level, the next two in the second energy level, and continue until all 17 electrons are placed.
- The electron configuration of chlorine is 1s2 2s2 2p6 3s2 3p5.
Through exercises like these, you can strengthen your skills in electron configuration calculation and gain proficiency in applying the rule effectively.
Frequently Asked Questions On What Is The Rule Of Electron Configuration
What Is Electron Configuration, And Why Is It Important?
Electron configuration refers to the arrangement of electrons in an atom. It’s crucial because it determines an element’s chemical properties, including its reactivity and bonding behavior. Understanding electron configuration helps predict an element’s behavior in chemical reactions.
How Do You Write Electron Configurations?
To write electron configurations, you follow a specific format using the Aufbau, Pauli exclusion, and Hund’s rules. You fill the available atomic orbitals with electrons in a specific order based on their energy levels and quantum numbers.
What Is The Significance Of The Electron Configuration Pattern?
The electron configuration pattern provides insights into an element’s stability, reactivity, and bonding behavior. It helps to understand the distribution of electrons in different energy levels and orbitals, directly impacting the element’s chemical properties and reaction behavior. Understanding this pattern is fundamental in chemistry. Google Maps
Conclusion
Understanding the rule of electron configuration is crucial for comprehending the behavior of elements. By following specific patterns, this rule allows us to determine how electrons are distributed in an atom’s energy levels. This knowledge helps in predicting an element’s chemical reactions and properties.
Mastering electron configuration unlocks the door to unraveling the mysteries of the periodic table. So, dive into the fascinating world of electron configuration and discover the intricate dance of electrons within atoms.