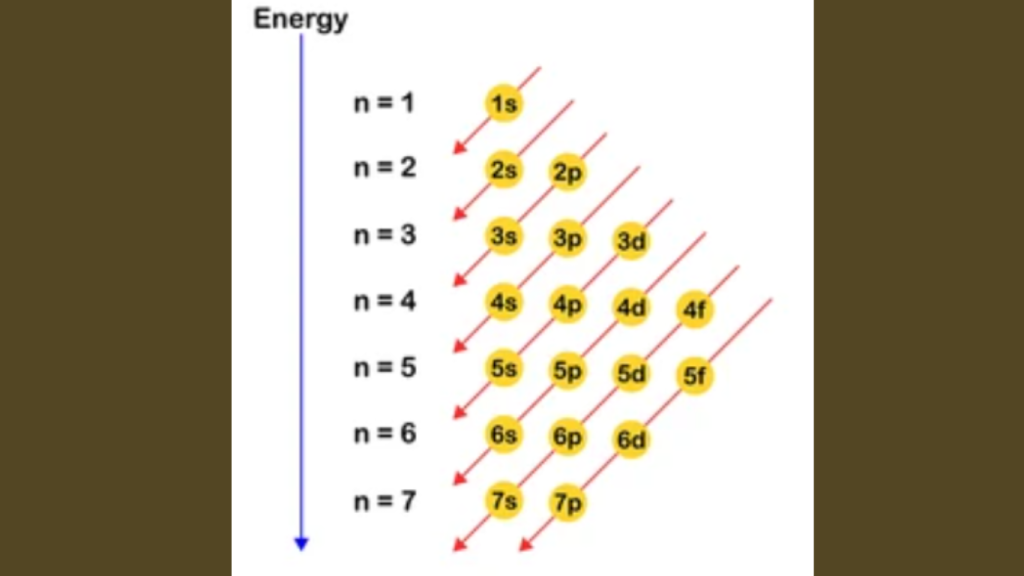
The purpose of electron configuration is to depict the arrangement of electrons in an atom’s energy levels and sublevels. It shows the distribution of electrons in different orbitals.
Understanding electron configuration is fundamental in comprehending an element’s chemical behavior and properties. By knowing the arrangement of electrons in an atom, scientists and chemists can predict how an element will react with other components, form chemical bonds, and determine its overall chemical reactivity.
Additionally, electron configuration provides valuable insights into an element’s stability and ability to form ions. This knowledge is crucial in various scientific fields, including chemistry, physics, and materials science. Furthermore, it serves as a foundation for understanding the periodic table and the patterns observed in the properties of elements.
Determining Electron Configuration
`Electron configuration is crucial in understanding an atom’s chemical behavior. It helps map out the electrons’ arrangement within an atom’s orbitals. The process is guided by specific principles known as the Aufbau Principle, Pauli Exclusion Principle, and Hund’s Rule.`
Aufbau Principle
`Electrons fill orbitals from lowest to highest energy levels. This principle guides the sequence of electron filling in atomic orbitals.`
Pauli Exclusion Principle
`States that no two electrons in an atom can have the same set of quantum numbers. This principle plays a vital role in determining electron arrangement. Google maps`
Hund’s Rule
`Electrons occupy orbitals singly before pairing up. This rule emphasizes that electrons prefer to maximize their spin and occupy different orbitals within the same subshell before pairing up.`
Frequently Asked Questions On What Is The Purpose Of Electron Configuration
What Is Electron Configuration?
Electron configuration is the arrangement of electrons in an atom’s energy levels. It helps to understand an element’s properties, including its reactivity and chemical bonding behavior.
Why Is Electron Configuration Important?
Understanding electron configuration is crucial for predicting an element’s chemical behavior, determining its reactivity, and explaining its placement in the periodic table.
How Do You Write Electron Configurations?
Electron configurations are written using the Aufbau principle, Pauli exclusion principle, and Hund’s rule, which determine the order and placement of electrons within an atom’s energy levels and sublevels.
Conclusion
Understanding electron configuration is crucial for comprehending elements’ behavior, properties, and interactions. By organizing electrons into specific energy levels, subshells, and orbitals, electron configuration provides a roadmap for chemical reactions and bonding. This knowledge allows scientists to predict an element’s reactivity and ability to form compounds.
Therefore, electron configuration is a fundamental concept in chemistry, enabling scientists to unlock the mysteries of the atomic world. Embrace this knowledge and delve deeper into the fascinating realm of chemistry.