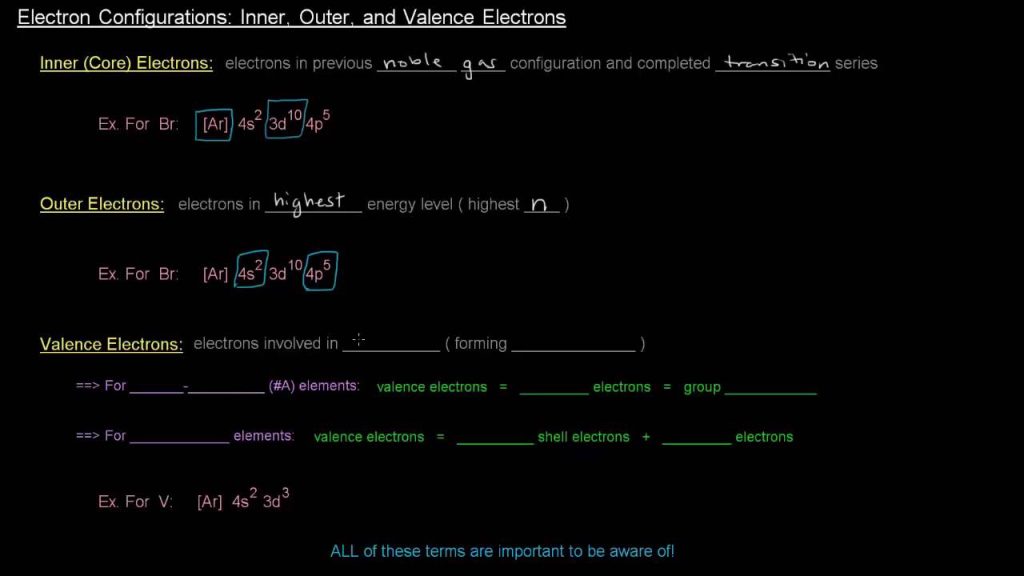
Electron configuration specifies the distribution of electrons in an atom, while valence electron configuration focuses on the outermost electron shell’s electrons. Understanding these concepts is vital in comprehending an element’s chemical behavior and reactivity.
Electron configuration indicates the precise arrangement of electrons amongst different energy levels and orbitals within an atom. On the other hand, valence electron configuration solely refers to the electrons in the outermost energy level of an atom, which is crucial in forming chemical bonds and determining an element’s properties.
By delving into electron and valence electron configurations, scientists can predict an element’s behavior in various reactions and understand its role in the periodic table.
Electron Configuration
Electron Configuration: Electron configuration refers to the arrangement of electrons within an atom, which is crucial for understanding an element’s properties.
Arrangement Of Electrons
In electron configuration, electrons occupy specific energy levels and orbitals around the nucleus of an atom.
- Electrons are arranged in shells, subshells, and orbitals based on energy levels.
- The distribution of electrons follows the Aufbau principle and the Pauli exclusion principle.
Representation In Periodic Table
The electron configuration of an element is reflected in its position on the periodic table.
| Group Number | Valence Electrons |
|---|---|
| 1 | 1 |
| 2 | 2 |
| … | … |
Importance In Chemistry
Understanding electron configuration is essential in predicting an element’s chemical reactivity and bonding behavior.
- It helps determine the possible oxidation states an element can exhibit.
- Electron configuration influences the element’s ability to form various types of chemical bonds.
Valence Electron Configuration
Valence electron configuration refers to the arrangement of electrons in the outermost energy level of an atom.
Location Of Valence Electrons
Valence electrons are found in the outermost energy level of an atom, also known as the valence shell.
Relationship To Chemical Reactivity
Valence electrons play a crucial role in determining the chemical reactivity of an atom.
Influence On Bonding
Valence electrons participate in the formation of chemical bonds between atoms.
Differences
Understanding the differences between electron configuration and valence electron configuration is crucial in comprehending the behavior and properties of elements. Let’s delve into the specific disparities in nature, their role in chemical behavior, and their application in predicting properties.
Nature Of Configuration
Electron configuration refers to the distribution of electrons in an atom’s orbitals, following a specific sequence based on the Aufbau principle, Pauli exclusion principle, and Hund’s rule. Valence electron configuration, on the other hand, denotes the arrangement of electrons in the outermost energy level, which determines an element’s reactivity and bonding capacity with other atoms.
Role In Chemical Behavior
The electron configuration dictates an element’s position in the periodic table, affecting its chemical behavior and reactivity. In contrast, the valence electron configuration primarily influences bonding tendencies, forming ionic, covalent, or metallic bonds and defining an element’s overall chemical behavior and interactions.
Application In Predicting Properties
Electron configuration aids in understanding an element’s stability, magnetic properties, and spectral characteristics. Alternatively, valence electron configuration is a valuable tool in predicting an element’s physical and chemical properties, such as its electronegativity, ionization energy, and tendency to form compounds.
Significance
The significance of understanding the difference between electron configuration and valence electron configuration lies in their fundamental role in comprehending the behavior and properties of elements in the periodic table. By delving into the impact on understanding elements, relevance in chemical reactions, and relationship to periodic trends, we can unravel the remarkable importance of these concepts.
Impact On Understanding Elements
The electron configuration provides invaluable insight into an element’s behavior. It indicates the distribution of electrons in different shells and subshells. Understanding the electron distribution, elements’ reactivity, and chemical properties can be predicted precisely.
Relevance In Chemical Reactions
Valence electron configuration determines an element’s ability to form compounds and participate in chemical reactions. It dictates the element’s valency and the type of bonds it can form, influencing the nature and strength of chemical interactions.
Relationship To Periodic Trends
The arrangement of electrons in both configurations directly influences an element’s position in the periodic table, contributing to the prediction of periodic trends such as ionization energy, electronegativity, and atomic size.
Examples
Here are examples illustrating the difference between Electron Configuration and Valence Electron Configuration:
Comparison Of Electron Configuration
Electron Configuration describes the distribution of electrons among atomic orbitals.
For example:
- Hydrogen has the electron configuration 1s1.
- Carbon has the electron configuration 1s2 2s2 2p2.
- Sodium has the electron configuration 1s2 2s2 2p6 3s1.
Comparison Of Valence Electron Configuration
Valence Electron Configuration focuses on the outermost shell electrons of an atom.
For example:
- Hydrogen has 1 valence electron (1s1).
- Carbon has 4 valence electrons (2s2 2p2).
- Sodium has 1 valence electron (3s1).
Importance In Chemical Bonding
The electron configuration and valence electron configuration play crucial roles in understanding atom behavior and chemical bonding. Each concept offers unique insights into the arrangement of electrons in an atom’s energy levels and their influence on the formation of chemical bonds. This understanding helps predict the nature of bonding and the resulting compounds, contributing significantly to the comprehension of chemical interactions.
Contribution To Formation Of Ionic Bonds
The electron and valence electron configurations are influential factors in forming ionic bonds. The electron configuration determines the distribution of electrons across different energy levels within an atom. Knowing the number of valence electrons makes it possible to ascertain an atom’s tendency to gain or lose electrons to achieve a stable configuration. This insight is vital in predicting the formation of ionic compounds, where atoms with low ionization energy lose electrons to those with high electron affinity, realizing stable noble gas configurations.
Influence On Covalent Bonding
Understanding valence electron configuration is vital in determining the potential for covalent bonding between atoms. By considering the number of valence electrons, it’s possible to predict the likelihood of atoms sharing electrons to attain stable configurations. The valence electron configuration reveals the electron-sharing tendencies of atoms, contributing to the prediction of molecular structures and the identification of polar and non-polar covalent bonds.
Frequently Asked Questions Of What Is The Difference Between Electron Configuration And Valence Electron Configuration
What Is Electron Configuration?
Electron configuration is the arrangement of electrons in an atom, molecule, or other physical system. It describes the distribution of electrons among energy levels and sublevels within an atom or ion, and this arrangement determines the atom’s chemical properties and behavior.
What Is Valence Electron Configuration?
Valence electron configuration refers to the arrangement of electrons in an atom’s outermost energy level, or valence shell. These electrons are involved in chemical bonding and determine the atom’s reactivity and ability to form bonds with other atoms. Google maps
What Is The Difference Between Electron Configuration And Valence Electron Configuration?
The main difference lies in which electrons are included in the configuration. Electron configuration describes the full distribution of electrons in all energy levels, while valence electron configuration focuses only on the electrons in the outermost energy level. Valence electron configuration is particularly important for understanding chemical bonding and reactivity.
Conclusion
Understanding the difference between electron configuration and valence electron configuration is essential in comprehending an element’s chemical properties. Mastering these concepts can aid in predicting an element’s reactivity, chemical bonding, and overall behavior. By grasping these fundamental principles, one can delve deeper into the intriguing world of chemistry with confidence and understanding.