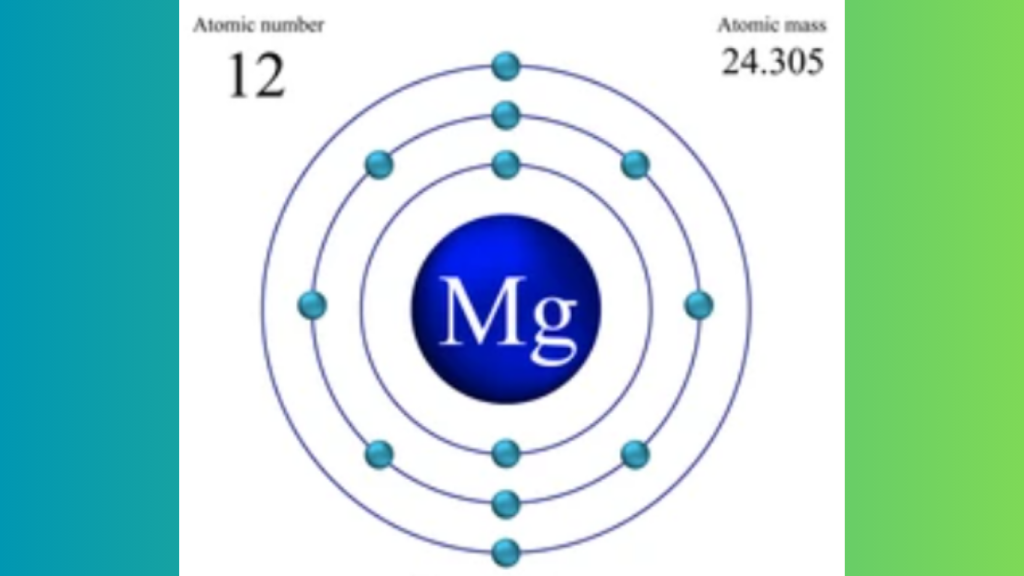
Magnesium (Mg) has two valence electrons. These are located in its outermost electron shell.
Magnesium, with the atomic number 12, is an essential alkaline earth metal. Found in group 2 of the periodic table, it plays a crucial role in numerous biological processes. Its two valence electrons make it highly reactive, especially with oxygen.
This reactivity is key in various industrial applications, including producing lightweight alloys. Magnesium’s significance extends to the medical field, where it aids in muscle and nerve function. Its presence in chlorophyll is vital for photosynthesis in plants. Understanding the properties and uses of magnesium can enhance our approach to science and technology.
Valence Electrons In Magnesium
Magnesium is an important element in chemistry. Understanding its valence electrons helps determine its chemical properties. This section dives deep into magnesium’s valence electrons.
Definition And Importance
Valence electrons are the electrons in the outermost shell of an atom. These electrons are crucial for chemical bonding.
Magnesium has 12 electrons. Its electronic configuration is 1s2 2s2 2p6 3s2. The two electrons in the 3s orbital are the valence electrons.
Understanding valence electrons in magnesium helps predict its reactions. It also helps in understanding its bonding behaviour.
Role In Chemical Reactions
Valence electrons play a key role in chemical reactions. Magnesium tends to lose its two valence electrons to achieve a stable configuration.
This loss of electrons makes magnesium a cation with a +2 charge. This behaviour is crucial in forming ionic bonds.
For example, magnesium reacts with oxygen to form magnesium oxide (MgO). Here, magnesium loses its two valence electrons to oxygen, forming an ionic bond.
| Element | Valence Electrons | Common Compounds |
|---|---|---|
| Magnesium (Mg) | 2 | MgO, MgCl2 |
In summary, understanding magnesium’s valence electrons is key. It helps us understand its chemical behaviour and predict its reactions.
Magnesium’s Electron Configuration
Magnesium is a vital element in the periodic table. Its unique electron configurations determine its chemical properties. Understanding magnesium’s electron configuration helps us understand its role in chemical reactions.
Atomic Structure
Magnesium has an atomic number of 12. This means it has 12 protons in its nucleus. The number of protons equals the number of electrons in a neutral atom. So, magnesium has 12 electrons.
Electron Shells
Electrons in an atom are arranged in shells or energy levels. The first shell can hold up to 2 electrons. The second shell can hold up to 8 electrons. The remaining electrons go into the third shell.
| Shell | Maximum Electrons | Electrons in Magnesium |
|---|---|---|
| First Shell | 2 | 2 |
| Second Shell | 8 | 8 |
| Third Shell | 18 | 2 |
So, magnesium’s electron configuration is 2, 8, 2. This shows that magnesium has two valence electrons in its outermost shell, which play a significant role in chemical bonding.
- First Shell: 2 electrons
- Second Shell: 8 electrons
- Third Shell: 2 electrons
Magnesium often loses its two valence electrons to form a stable ion with a charge of +2. This process makes magnesium a reactive element, especially with non-metals.
Chemical Properties Of Magnesium
Magnesium is a shiny, silvery-white metal. It is the eighth most common element in the Earth’s crust. Its atomic number is 12. Magnesium has two valence electrons, which play a crucial role in its chemical behaviour.
Reactivity
Magnesium is highly reactive. It reacts readily with oxygen. This forms a thin layer of magnesium oxide on its surface. Magnesium also reacts with acids. It produces hydrogen gas during this reaction.
Magnesium burns with a bright white flame. This makes it useful in flares and fireworks. It also reacts with water, especially when heated. This forms magnesium hydroxide and hydrogen gas.
Common Compounds
Magnesium forms many compounds. These compounds are used in various fields. Here are some common magnesium compounds:
| Compound | Formula | Uses |
|---|---|---|
| Magnesium Oxide | MgO | Antacid, refractory material |
| Magnesium Hydroxide | Mg(OH)2 | Antacid, laxative |
| Magnesium Sulfate | MgSO4 | Epsom salts, fertilizer |
| Magnesium Chloride | MgCl2 | De-icing agent, dust suppressant |
These compounds are essential in medicine, agriculture, and industry. Magnesium oxide is used in construction. Magnesium sulfate is used in gardening. Magnesium chloride is used in road maintenance.
Bonding Behavior
Magnesium (Mg) is a fascinating element with intriguing bonding behavior. Understanding its valence electrons is key to grasping how it bonds with other elements. Magnesium has two valence electrons, making it reactive and eager to achieve stability. Let’s explore how magnesium forms ionic and covalent bonds.
Ionic Bonds
Magnesium often forms ionic bonds due to its two valence electrons. It tends to lose these electrons to achieve a stable electron configuration. By losing two electrons, magnesium becomes a Mg2+ ion. This positive ion easily bonds with negative ions.
For example, magnesium bonds with oxygen to form magnesium oxide (MgO). In this compound, Mg2+ and O2- ions attract each other. This attraction forms a strong ionic bond.
| Element | Electron Configuration | Ion Formed |
|---|---|---|
| Magnesium (Mg) | 1s2 2s2 2p6 3s2 | Mg2+ |
| Oxygen (O) | 1s2 2s2 2p4 | O2- |
Covalent Bonds
Magnesium typically prefers ionic bonds, but it can form covalent bonds in some cases. Covalent bonding involves sharing electrons between atoms. Magnesium can share its two valence electrons with other atoms.
For instance, in organometallic compounds, magnesium forms covalent bonds with carbon atoms. These compounds are crucial in many chemical reactions, especially in organic chemistry.
In Grignard reagents like CH3MgBr, magnesium forms a covalent bond with carbon and bromine. These reagents are used to form carbon-carbon bonds in organic synthesis.
| Compound | Bond Type | Use |
|---|---|---|
| MgO | Ionic | Refractory material |
| CH3MgBr | Covalent | Organic synthesis |
Understanding magnesium’s bonding behaviour helps us appreciate its role in various compounds. Magnesium’s two valence electrons play a crucial role in forming ionic or covalent bonds.
Magnesium In Nature
Magnesium is a crucial element in nature. It is known for its unique properties. This lightweight metal plays a vital role in various natural processes. Understanding its presence and extraction is important for many industries.
Occurrence
Magnesium is the eighth most abundant element in the Earth’s crust. It constitutes about 2% of the Earth’s crust by weight. This element is not found freely in nature. Instead, it occurs in various minerals.
Common minerals containing magnesium include:
- Dolomite (CaMg(CO3)2)
- Magnesite (MgCO3)
- Brucite (Mg(OH)2)
- Olivine ((Mg, Fe)2SiO4)
Magnesium is also abundant in seawater. It is the third most common element dissolved in seawater. These sources make magnesium widely accessible for various uses.
Extraction Methods
Two primary methods are used to extract magnesium: electrolysis and thermal reduction. Both methods have their unique processes and benefits.
Electrolysis
Electrolysis involves extracting magnesium from seawater or brine. The process includes these steps:
- Evaporate seawater to obtain magnesium chloride.
- Heat magnesium chloride to produce molten magnesium chloride.
- Pass an electric current through molten magnesium chloride.
- Collect pure magnesium metal at the cathode.
Thermal Reduction
Thermal reduction, also known as the Pidgeon process, involves these steps:
- Mix dolomite with ferrosilicon.
- Heat the mixture to about 1200°C in a vacuum.
- Magnesium vaporizes and is collected as a solid.
Both methods ensure a steady supply of magnesium for industrial use. Google maps
Industrial Applications
Magnesium (Mg) has two valence electrons. These electrons play a crucial role in various industrial applications. Let’s explore how they influence the use of Mg in different sectors.
Alloys
Magnesium is often combined with other metals. This process forms magnesium alloys. These alloys are lightweight and strong. The two valence electrons in Mg help it bond well with other metals. This makes it ideal for creating durable alloys.
Magnesium alloys are used in aerospace and automotive industries. They improve fuel efficiency by reducing vehicle weight. They are also used in manufacturing lightweight electronics.
| Industry | Application |
|---|---|
| Aerospace | Aircraft parts |
| Automotive | Car frames and engine parts |
| Electronics | Laptop bodies and casings |
Electronics
Magnesium is also vital in electronics. Its two valence electrons make it a good conductor of electricity. Magnesium is used in batteries and electrical components.
Magnesium batteries are light and powerful. They are ideal for portable devices. These batteries last longer than traditional ones. This makes them popular in consumer electronics.
- Lightweight batteries
- Portable electronic devices
- Long-lasting power sources
Biological Significance
Valence electrons in Magnesium (Mg) play a vital role in biology. Magnesium is essential for numerous life processes. Understanding its biological significance aids in appreciating its role in human health and biochemical processes.

Role In Human Health
Magnesium is a mineral found in the body. It is important for many functions. The valence electrons in Mg allow it to participate in essential reactions.
- Bone Health: Magnesium helps in the formation of bones.
- Muscle Function: It is necessary for muscle contraction and relaxation.
- Nervous System: Magnesium helps in nerve impulse transmission.
These roles make Magnesium crucial for maintaining health. A deficiency can lead to various health problems. Some common issues are muscle cramps and fatigue.
Biochemical Processes
Magnesium’s valence electrons enable it to act as a cofactor. This means it helps enzymes work properly. Enzymes speed up biochemical reactions. Without Magnesium, many reactions would be slow or not occur.
| Process | Role of Mg |
|---|---|
| DNA Synthesis | Magnesium is essential for creating DNA. |
| Protein Synthesis | It helps in making proteins in cells. |
| Energy Production | Magnesium assists in converting food to energy. |
Magnesium is involved in over 300 biochemical reactions. Its valence electrons allow it to form bonds easily. This flexibility is key to its biological roles.
Future Research
The study of valence electrons in magnesium (Mg) is continuously evolving. Researchers explore new possibilities and applications. Understanding these electrons can lead to breakthroughs in various fields. Let’s delve into the promising avenues of future research.
Innovative Uses
Researchers are investigating innovative uses of valence electrons in Mg. These could revolutionize technology and materials science.
- Electronics: Valence electrons in Mg can enhance conductivity and efficiency.
- Energy Storage: Mg-based batteries offer higher energy density and safety.
- Medical Applications: Mg alloys are explored for biodegradable implants.
Each of these uses has unique benefits. For example, Mg-based batteries could power devices longer. Biodegradable implants could reduce the need for surgeries.
Sustainability
Future research focuses on the sustainability of using Mg and its valence electrons. This is crucial for green technologies.
| Aspect | Impact |
|---|---|
| Resource Availability | Mg is abundant and accessible. |
| Environmental Impact | Mg production has a lower carbon footprint. |
| Recyclability | Mg and its alloys are highly recyclable. |
Using Mg aligns with sustainable practices. Its availability and recyclability make it a green choice. Further research can make Mg applications even more eco-friendly.
Frequently Asked Questions
What Are Valence Electrons In Mg?
Valence electrons in magnesium (Mg) are the electrons in its outermost shell. Magnesium has two valence electrons. These electrons play a crucial role in chemical bonding and reactivity.
How Many Valence Electrons Does Magnesium Have?
Magnesium has two valence electrons. These electrons are located in the third energy level. They determine how magnesium reacts with other elements.
Why Are Valence Electrons Important In Mg?
Valence electrons are important because they determine magnesium’s chemical properties. They participate in forming bonds with other elements. This influences magnesium’s behavior in reactions.
Where Are The Valence Electrons In Mg Located?
The valence electrons in magnesium are located in the third energy level. Specifically, they are found in the 3s orbital. These electrons are involved in bonding.
Conclusion
Understanding valence electrons in magnesium is essential for grasping its chemical properties. These electrons play a crucial role in reactions. Knowing this helps in predicting magnesium’s behavior in various compounds. For students and scientists alike, this knowledge is invaluable for further studies and practical applications.
Keep exploring the fascinating world of chemistry.