We will now discuss the octet rule and oxygen’s electron configuration. The octet rule states that atoms seek eight electrons in their outer shell. Oxygen’s electron configuration is 1s² 2s² 2p⁴.
The octet rule is a fundamental concept in chemistry. It helps explain why atoms form certain chemical bonds. The octet rule and oxygen’s electron configuration essential element that closely follows this rule. Its electron configuration of 1s² 2s² 2p⁴ means it has six electrons in its outer shell.
The octet rule and oxygen’s electron configuration are heavily involved in this theory. To achieve a full octet, oxygen gains or shares two electrons. This behavior makes it highly reactive and essential for life. Understanding oxygen’s electron configuration is crucial for grasping its role in chemical reactions. It plays a vital role in forming compounds like water and carbon dioxide.
Introduction To The Octet Rule
The octet rule is key in chemistry. It helps explain how atoms bond. Atoms want to have eight electrons in their outer shell, which makes them more stable. Chemists use the octet rule to predict molecule shapes and understand chemical reactions.
Atoms bond to get eight electrons to follow the octet rule. They may share, lose, or gain electrons. For example, Oxygen’s electron configuration has six electrons in its outer shell. Two more are needed to complete the octet rule. Oxygen often forms bonds to fill this need. This is why oxygen is so reactive.
Role Of Valence Electrons
Why are valence electrons important? Valence electrons are the outermost electrons of an atom. These electrons determine how an atom reacts with others. Atoms with a full valence shell are stable. Most atoms aim to have eight valence electrons. This is called the octet rule. Atoms will gain, lose, or share electrons to achieve this.
Oxygen has six valence electrons. To satisfy the octet rule, two more electrons are needed. Oxygen often forms bonds with other elements to get these electrons. For example, it can bond with two hydrogen atoms to form water (H₂O). Another example is bonding with another oxygen atom to form O₂, which we breathe.
Here’s a showing of the number of simplified table of elements with valence electrons for common elements. The number of valence electrons corresponds to the electrons in the outermost electron shell, which plays a key role in chemical bonding:
| Element | Symbol | Group | Number of Valence Electrons |
|---|---|---|---|
| Hydrogen | H | 1 | 1 |
| Helium | He | 18 | 2 |
| Lithium | Li | 1 | 1 |
| Beryllium | Be | 2 | 2 |
| Boron | B | 13 | 3 |
| Carbon | C | 14 | 4 |
| Nitrogen | N | 15 | 5 |
| Oxygen | O | 16 | 6 |
| Fluorine | F | 17 | 7 |
| Neon | Ne | 18 | 8 |
| Sodium | Na | 1 | 1 |
| Magnesium | Mg | 2 | 2 |
| Aluminum | Al | 13 | 3 |
| Silicon | Si | 14 | 4 |
| Phosphorus | P | 15 | 5 |
| Sulfur | S | 16 | 6 |
| Chlorine | Cl | 17 | 7 |
| Argon | Ar | 18 | 8 |
| Potassium | K | 1 | 1 |
| Calcium | Ca | 2 | 2 |
| Gallium | Ga | 13 | 3 |
| Germanium | Ge | 14 | 4 |
| Arsenic | As | 15 | 5 |
| Selenium | Se | 16 | 6 |
| Bromine | Br | 17 | 7 |
| Krypton | Kr | 18 | 8 |
Notes:
- Group 1 elements (alkali metals) have 1 valence electron.
- Group 2 elements (alkaline earth metals) have 2 valence electrons.
- Groups 13-18 represent nonmetals, metalloids, and noble gases, with valence electrons ranging from 3 to 8, based on their group number minus 10.
Noble gases like Neon, Argon, and Krypton are stable because they have 8 valence electrons (except Helium, which has 2).
Oxygen’s Atomic Structure
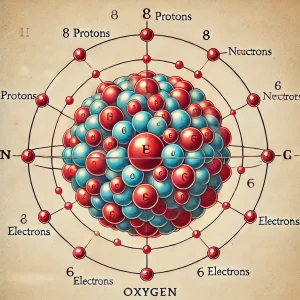
From Oxygen’s atomic structure It has 8 protons in its nucleus. This gives Oxygen atomic number 8. Oxygen also has 8 neutrons in its nucleus. Together, protons and neutrons give the oxygen atomic mass of 16. Oxygen has 8 electrons orbiting its nucleus. These electrons are arranged in energy levels.
Oxygen is in group 16 of the periodic table. It is in period 2. Oxygen is a non-metal and is very reactive. It is essential for life on Earth. Oxygen is the third most abundant element in the universe.
Electron Configuration Of Oxygen
Oxygen has 8 electrons. Electrons fill orbitals in a specific order. The first two electrons fill the 1s orbital. This is the closest orbital to the nucleus. The next two electrons fill the 2s orbital. The remaining four electrons fill the 2p orbital. These electrons spread out to minimize repulsion.
Oxygen’s electrons have two energy levels. The first level has the 1s orbital, which holds two electrons. The second energy level has the 2s and 2p orbitals, which hold two electrons and the remaining four electrons. The 2p orbital is higher in energy than the 2s orbital.
Octet Rule In Oxygen
Oxygen has six electrons in its outer shell. To achieve stability, it needs two more electrons. This follows the octet rule, which means having eight electrons in the outer shell. By gaining or sharing electrons, oxygen becomes stable. That’s why from Oxygen’s electron configuration we can learn the Octet rule formula very well.
Oxygen often forms compounds with hydrogen and carbon, including water (H2O) and carbon dioxide (CO2). In water, oxygen shares electrons with hydrogen, and in carbon dioxide, it shares electrons with carbon. Those are the proof of the Oxygenn’s Octet rule formula.
Exceptions To The Octet Rule
Some elements can have more than eight electrons, a phenomenon called an expanded octet. Sulfur and phosphorus are examples. These elements can hold more electrons because they have larger atomic sizes, which provide more space for extra electrons, helping them form more bonds.
Some elements do not need eight electrons, known as incomplete octets. Boron and hydrogen are common examples. Boron often has only six electrons, while hydrogen is stable with just two electrons. These exceptions make them unique.
Chemical Bonding And Oxygen
Chemical bonding refers to the interaction between atoms that enables the formation of chemical compounds. These bonds hold atoms together in molecules or crystals and arise from the sharing, transfer, or attraction of electrons between atoms. There are 3 types of chemical bonds.
1. Covalent bonds
2. Ionic bonds
3. Metallic bonds
Oxygen atoms not able to performing 3 types of chemical bonds. But It can able to perform 2 types of chemical bonds.
Oxygen atoms form covalent bonds by sharing electrons. Each oxygen atom needs two more electrons to complete its octet. By sharing, each atom gets to feel like it has eight electrons. This makes the atoms more stable.
Oxygen can also form ionic bonds with other elements. In ionic bonds, oxygen gains two electrons from another atom. This makes oxygen a negative ion. The other atom becomes a positive ion. These opposite charges attract, holding the atoms together.
Applications In Real Life
Understanding the octet rule and oxygen’s electron configuration helps explain chemical bonding in everyday compounds like water and carbon dioxide. This principle is fundamental in predicting molecule stability and reactions in various real-life applications.
Learn more about Oxygen Electron Configuration in the main guide
Frequently Asked Questions
What Is The Octet Rule?
The octet rule states that atoms are most stable with eight electrons in their valence shell. This configuration is similar to noble gases.
How Does Oxygen Follow The Octet Rule?
Oxygen follows the octet rule by gaining two electrons. This gives it a complete set of eight valence electrons.
What Is Oxygen’s Electron Configuration?
Oxygen’s electron configuration is 1s² 2s² 2p⁴. This means it has two electrons in the first shell and six in the second.
Why Is The Octet Rule Important?
The octet rule is important because it explains the stability of atoms. Atoms bond to achieve a full valence shell.
Conclusion
Understanding the octet rule and oxygen’s electron configuration is essential for grasping chemical bonding. These concepts explain how atoms achieve stability. By mastering these basics, you can better understand more complex chemical reactions. Keep exploring to deepen your knowledge of chemistry’s fundamental principles. Google Maps