The electron configuration of tellurium is [Kr] 4d10 5s2 5p4, which means it has 2 electrons in the outermost shell. Tellurium is a metalloid element with the atomic number 52, and it belongs to the group of chalcogens in the periodic table.
Tellurium has a greyish-white appearance and is commonly used in alloys, semiconductors, and solar panels. It also exhibits photovoltaic and thermoelectric properties, making it a promising material for renewable energy technologies. We will discuss the electron configuration of tellurium in detail and explore its properties and uses.
The Electron Configuration Of Tellurium
Tellurium’s electron configuration is [Kr] 4d10 5s2 5p4. It has six valence electrons in its outermost shell, making it a member of the oxygen family. This semi-metal is known for its semiconductor and metalloid properties.
What Is Electron Configuration?
Electron configuration refers to the arrangement of electrons in an atom’s energy levels or shells. Electrons occupy the lowest energy shells first before moving to the higher levels. An atom’s electron configuration determines its chemical and physical properties, including ionization energy, electronegativity, and reactivity.
What Is Tellurium’s Electron Configuration?
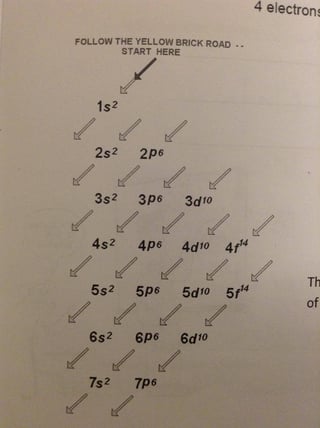
Tellurium is a chemical element with the symbol Te and atomic number 52. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p4, where the 1s2 level holds two electrons, and the 2s2, 2p6, 3s2, 3p6, 3d10, 4s2, 4p6, 4d10, and 5s2 levels can hold up to 18 electrons each.
Understanding Tellurium’s Shells And Subshells
Tellurium is a p-block element with five valence electrons in the 5s and 5p orbitals. Its electron configuration shows a filled 4d10 subshell, which contributes to its high melting and boiling points. Additionally, the 5p subshell has only four electrons, making tellurium electron-deficient, contributing to its reactivity.
Why Is Tellurium’s Electron Configuration Important?
Knowing the electron configuration of tellurium or any other element can help predict its chemical behavior. By looking at the number of valence electrons, you can determine whether an atom is more likely to gain or lose electrons in a chemical reaction. Additionally, understanding the subshells and energy levels that an element’s electrons occupy can help explain its unique chemical and physical properties.
Properties Of Tellurium
Tellurium is a rare metalloid element with an electron configuration of [Kr]4d10 5s2 5p4. It is brittle, silvery-white, and semiconducting in nature. It is used in alloys, solar panels, and the electronics industry.
Tellurium is a metalloid element with the atomic number 52 and the symbol Te. It is a brittle, silver-gray substance that belongs to the chalcogen group on the periodic table, including sulfur and selenium. Tellurium primarily produces alloys, solar panels, and some electronic devices. Let’s take a closer look at the properties of Tellurium.
Physical Properties
Tellurium has unique physical properties that make it stand out from other elements. Here are some of its physical properties:
-
Tellurium has a density of 6.24 g/cm3.
-
It is a brittle, lustrous metalloid that exhibits a silvery-gold color when found in its pure form.
-
Its melting point is 722.66 Kelvin, and its boiling point is 1,261 Kelvin.
-
It is a semiconductor, which means that it can conduct some electricity under certain conditions.
Chemical Properties
Tellurium has a unique set of chemical properties that make it valuable in various industrial applications. Here are some of its chemical properties:
-
Tellurium has six stable isotopes.
-
It reacts with oxygen, halogens, and acids to form various tellurium oxides, halides, and salts.
-
It is mildly toxic and can cause skin irritation and respiratory problems when inhaled.
-
Tellurium can form alloys with several metals, including lead, copper, and silver.
Significance Of Tellurium’s Properties
Tellurium’s properties make it an essential element in various industrial applications. Here are some ways in which its properties are significant:
|
Application |
Significance of Tellurium’s Properties |
|---|---|
|
Alloys for Solar Panels |
Tellurium’s semiconductor properties make it an ideal component for alloys used in solar panels, as it can convert sunlight directly into electricity. |
|
Electronics |
Tellurium’s ability to form alloys with other metals makes it valuable in producing various electronic devices, such as CDs and DVDs. |
|
Metallurgy |
It is used in metallurgy to improve the properties of metals when used as an alloying agent. |
|
Chemical Industry |
Tellurium compounds are used to produce rubber and other chemically resistant materials. |
In conclusion, Tellurium’s unique physical and chemical properties make it an essential element in many industrial applications. Its properties are vital in producing solar panels, electronic devices, and other chemical industries.
Applications Of Tellurium
Tellurium’s electron configuration allows it to be used in various applications. It is commonly used to produce alloys, semiconductors, and solar panels. Additionally, it has antibacterial properties and can be found in some medicinal products.
Tellurium is a brittle, silver-white metalloid that belongs to the selenium group. It has numerous applications in metallurgy, solar energy, and other industries. Here we explore some of these applications in detail:
Metallurgy And Alloys
Tellurium is widely used to manufacture free-machining steels, cast iron, and copper alloys. This is because it improves the machinability and durability of these alloys. It is also used to produce lead-free solders, which are essential in the electronics industry as they have lower toxicity levels than lead solders. Moreover, tellurium can be added to lead or copper electrodes to increase their resistance to corrosion and improve their lifespan.
Solar Energy
Tellurium is a vital component in producing solar cells, and its usage has recently increased due to the demand for renewable energy sources. When combined with cadmium and sulfur, tellurium forms cadmium telluride, a photoelectric material used in thin-film solar cells. Solar cells made of cadmium telluride are highly efficient and can absorb sunlight more effectively than traditional silicon-based solar cells.
Other Uses
Apart from metallurgy and solar energy, tellurium has other applications. Some of these applications include:
-
Tellurium is an essential ingredient in blasting caps and detonators used in the explosives industry.
-
It is used to produce thermoelectric materials used in power generation technology.
-
It is used as a coloring agent in glass and ceramics industries, producing various colors such as ruby red and green.
In conclusion, tellurium is crucial in various industries, such as metallurgy and solar energy. Its unique properties make it an indispensable element in the manufacturing of numerous alloys, solar cells, and other products.
Frequently Asked Questions For Tellurium Electron Configuration
How Do You Write The Electron Configuration For Tellurium?
To write the electron configuration for tellurium, use the Aufbau principle and place electrons in increasing order of energy levels and sublevels. Tellurium has 52 electrons, so the configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p4.
What Element Has An Electron Configuration Of 1s 2 2s 2 2p 6 3s 2 3p 4?
The element with an electron configuration of 1s2 2s2 2p6 3s2 3p4 is sulfur.
What Is The Electron Configuration Of Ts 117?
The electron configuration of Ts 117 is [Rn] 5f14 6d10 7s2 7p5.
What Element Is The Following Electron Configuration For Ar 4s23d6?
The given electron configuration is for Argon, which has 18 protons in its nucleus. The configuration indicates that it has 2 electrons in the 4th shell (4s2) and 6 electrons in the 3rd shell (3d6).
Conclusion
Overall, understanding the electron configuration of tellurium is essential for appreciating its unique properties and behaviors. Scientists and researchers can predict its chemical reactions and even apply its potential applications in various industries by being aware of the arrangement of electrons in tellurium atoms.
While it may seem complex initially, mastering this concept is an important step toward advancing our knowledge of the elements we use daily. Keep exploring and learning about the fascinating world of chemistry!