How Do You Know If an Electron Configuration is in Ground State
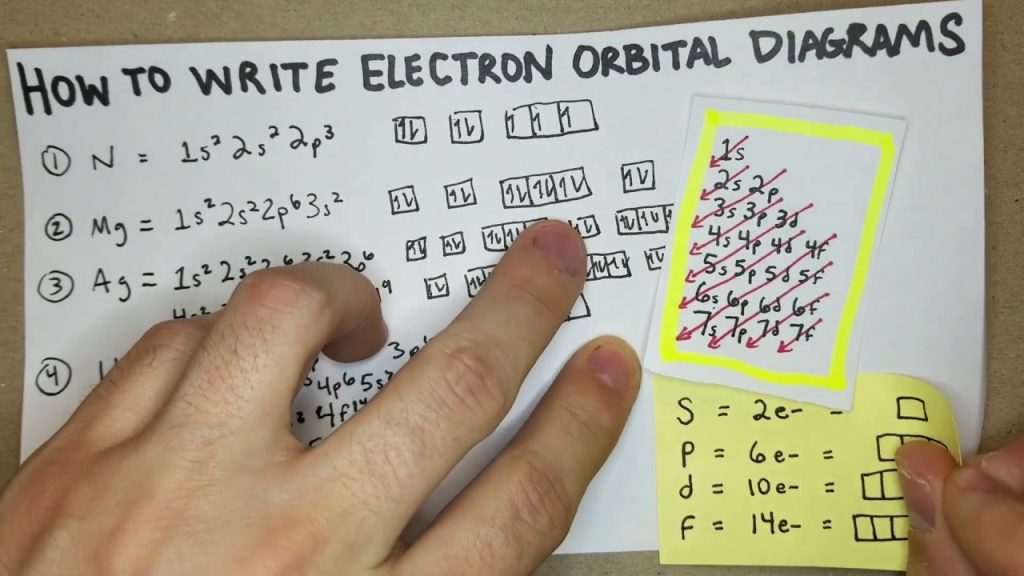
In the ground state, an electron configuration is at its lowest energy level, with all electrons in their designated orbitals. This is determined by following the Aufbau principle and the Pauli exclusion principle. Understanding the ground state of an electron configuration is essential in chemistry to predict chemical reactions and properties of elements. Scientists can […]
How Do You Know If an Electron Configuration is in Ground State Read More »