Now, we discuss Oxygen’s valence electrons and their role in chemical bonding. Oxygen has six valence electrons, which are crucial in forming chemical bonds.
Oxygen, atomic number 8, is an essential element in the periodic table. It is highly electronegative and readily bonds with other elements to achieve a stable electron configuration. The six valence electrons in oxygen allow it to form up to two covalent bonds, sharing electrons with other atoms to complete its octet.
This bonding capability makes oxygen a key component in water (H2O), organic compounds, and numerous chemical reactions. Understanding oxygen’s valence electrons and bonding behavior is fundamental in chemistry, impacting biological processes and industrial applications. Oxygen’s versatile bonding nature enables it to participate in various chemical reactions, making it indispensable in both nature and technology.
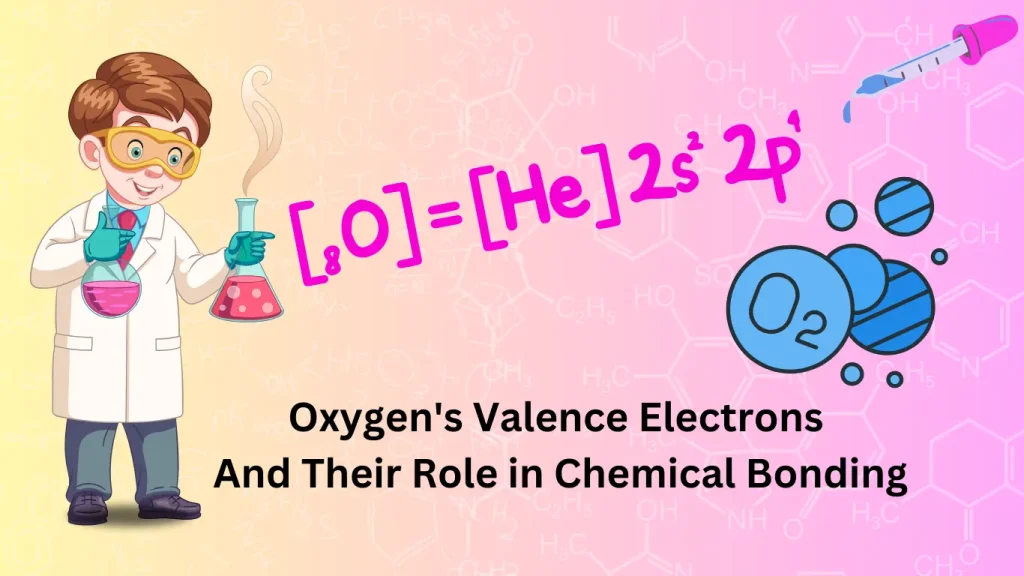
Introduction To Oxygen’s Valence Electrons
Oxygen has eight electrons. Electrons circle the nucleus in shells. The first shell holds two electrons. The second shell holds six electrons. Oxygen has six electrons in the second shell. These are called valence electrons.
Oxygen’s valence electrons are in the second shell. They help in chemical bonding. Oxygen forms bonds by sharing electrons. This makes it stable. Valence electrons are important in reactions. They determine how atoms bond with each other.
Importance In Chemical Bonding
Oxygen has six valence electrons, which are important for bonding. Atoms bond to achieve a full shell of electrons, and oxygen needs two more electrons to complete its shell.
In bonding, oxygen shares or takes electrons from other atoms. This sharing makes atoms more stable, and stable atoms form strong bonds, which make molecules stable.
Oxygen often forms covalent bonds. In covalent bonds, atoms share electrons. Each atom gets a full shell of electrons. Oxygen can form two covalent bonds. This is because it needs two more electrons.
Oxygen often bonds with hydrogen. This forms water molecules. Each hydrogen atom shares one electron with oxygen. This sharing fills oxygen’s shell and makes water stable.
Octet Rule And Oxygen
Oxygen has six valence electrons. It needs two more electrons to achieve stability. By gaining or sharing electrons, oxygen completes its octet. This makes oxygen more stable. Oxygen often forms bonds with other elements. These bonds help oxygen achieve a full outer shell. A full outer shell has eight electrons. This is called the octet rule.
Some molecules do not follow the octet rule. Oxygen can sometimes form compounds without eight electrons. These cases are exceptions. They are rare but important to know. Free radicals are an example. They have unpaired electrons, which makes them very reactive. Understanding these exceptions helps in studying chemistry.
Oxygen In Organic Compounds
Oxygen is key in many functional groups, including alcohols, ethers, and carboxylic acids. Each group has unique properties due to oxygen’s presence. Alcohols have an -OH group. Ethers contain oxygen between two carbon atoms. Carboxylic acids have a -COOH group, making them acidic. Oxygen’s valence electrons help form these bonds.
Oxygen atoms form hydrogen bonds with hydrogen atoms. These bonds are weak but very important. Water molecules stick together because of hydrogen bonds. They also affect the structure of proteins and DNA. Hydrogen bonding gives water a high boiling point. Oxygen’s valence electrons attract hydrogen atoms, forming these bonds.
Oxygen In Inorganic Chemistry
Oxygen forms oxides by bonding with other elements. In oxides, oxygen usually has a -2 charge. Common oxides include water (H2O) and carbon dioxide (CO2). Oxides are important in many chemical reactions.
Peroxides contain an oxygen-oxygen single bond. Hydrogen peroxide (H2O2) is a well-known example. Peroxides have oxygen with a -1 charge. Superoxides have one oxygen molecule with a -1/2 charge. Potassium superoxide (KO2) is used in breathing masks. Superoxides are more reactive than oxides and peroxides.
Oxygen’s Role In Biological Systems
Oxygen is essential for cellular respiration. Cells use oxygen to produce energy, called ATP. Oxygen helps break down glucose in the mitochondria. Without oxygen, cells cannot make enough energy, leading to fatigue and other problems.
Plants use oxygen in photosynthesis. This process makes food for the plant. Plants take in carbon dioxide and give off oxygen. This oxygen is important for animals and humans. It helps us breathe and stay alive. Plants and oxygen are very important for life on Earth.
Common Oxygen Compounds
Oxygen’s six valence electrons make it highly reactive, forming compounds like water and carbon dioxide. Its ability to form stable bonds is crucial in various chemical reactions.
Water (H2O)
Water is made of two hydrogen atoms and one oxygen atom. Oxygen has six valence electrons. It needs two more electrons to be stable. Hydrogen has one valence electron. Each hydrogen atom shares its electron with oxygen. This sharing creates a strong bond. Water is a liquid at room temperature. It is essential for life.
Carbon Dioxide (CO2)
Carbon dioxide has one carbon atom and two oxygen atoms. Carbon has four valence electrons. Oxygen needs two more electrons to be stable. Each oxygen shares two electrons with carbon. This forms a double bond. Carbon dioxide is a gas at room temperature. It is used by plants for photosynthesis.
Advanced Bonding Concepts
Oxygen has six valence electrons. These electrons play a key role in bonding. Molecular orbital theory explains how these electrons combine with others. When atoms bond, they form molecular orbitals. These orbitals can hold electrons from both atoms. Electrons fill the lower energy orbitals first. This makes the molecule more stable. Bonding and anti-bonding orbitals are important concepts. Bonding orbitals lower the energy. Anti-bonding orbitals raise it. Understanding this theory helps us know how oxygen bonds.
Hybridization is another important concept. It helps explain the shapes of molecules. Oxygen uses sp3 hybridization in water. This means one s orbital and three p orbitals mix, forming four sp3 hybrid orbitals. Two of these hold lone pairs of electrons. The other two form bonds with hydrogen, giving the water its bent shape. Hybridization helps us understand many molecular structures. It is a key part of chemical bonding. Learn more about Oxygen Electron Configuration in the main guide
Applications In Industry
Oxygen plays a key role in steel production. It helps remove impurities like carbon and sulfur. This process makes the steel stronger and more durable. Oxygen is injected into molten iron. This causes unwanted elements to form oxides. These oxides are then removed from the steel. This improves the quality of the final product.
Oxygen is vital in rocket propellants. It acts as an oxidizer, which means it helps fuel burn more efficiently. Liquid oxygen is often used in rockets. It combines with hydrogen to create thrust, which allows rockets to reach space. Without oxygen, rockets would not have enough power to leave Earth.
Frequently Asked Questions
What Are Valence Electrons In Oxygen?
Valence electrons are the outermost electrons in an atom. In oxygen, there are six valence electrons. These electrons play a key role in chemical bonding.
How Do Oxygen’s Valence Electrons Form Bonds?
Oxygen’s six valence electrons allow it to form bonds by sharing or gaining electrons, resulting in stable chemical compounds.
Why Is Oxygen Highly Reactive?
Oxygen is highly reactive because it needs two more electrons to complete its outer shell. This makes it eager to bond with other elements.
What Type Of Bonds Does Oxygen Form?
Oxygen commonly forms covalent bonds by sharing electrons. It can also form ionic bonds by gaining electrons from metals.
Conclusion
Understanding oxygen’s valence electrons is crucial in chemical bonding. These electrons determine how oxygen interacts with other elements, forming bonds that create essential compounds. Mastering this concept unlocks the fundamentals of chemistry. Keep exploring to deepen your knowledge of chemical interactions and their real-world applications. Google Maps