Oxygen is one of the most essential elements for life on Earth, and understanding its electron configuration helps us grasp its chemical behavior. The electron configuration of an atom describes how its electrons are distributed in orbitals around the nucleus. In this article, we’ll explore the electron configuration of oxygen, how it’s determined, and why it’s significant in both chemical bonding and reactions.
What is Electron Configuration?
Electron configuration is the arrangement of electrons in an atom’s orbitals, following specific rules that govern their distribution. These rules include:
The Aufbau Principle: Electrons fill orbitals starting from the lowest energy level and work their way up.
The Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons with opposite spins.
Hund’s Rule: Electrons will singly occupy orbitals of the same energy level before pairing up.
These rules allow us to predict where each electron in an atom is located, which is crucial for understanding how that atom interacts with others.
Oxygen’s Atomic Structure
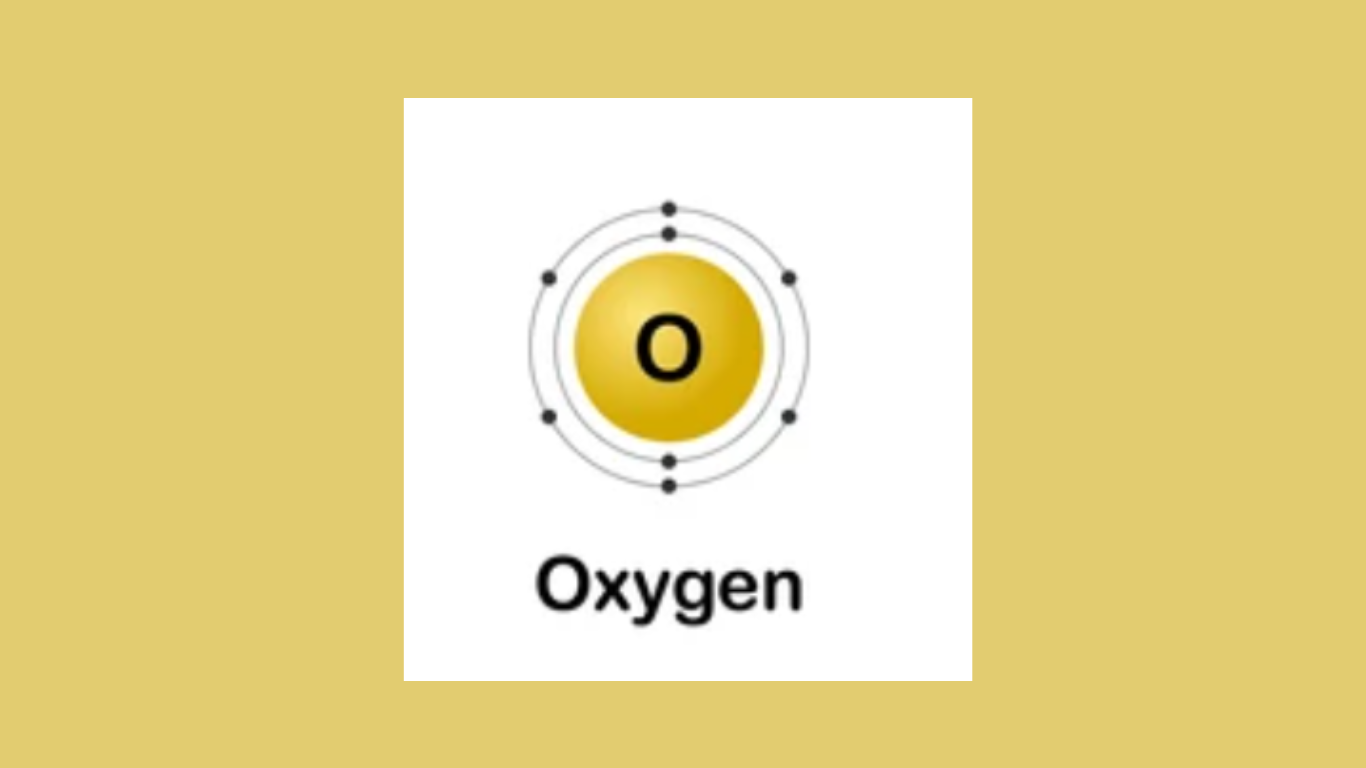
Oxygen is a member of the chalcogen group on the periodic table and has an atomic number of 8. This means it has eight protons in its nucleus and, in a neutral atom, an equal number of electrons. These electrons are distributed around the nucleus across different energy levels or shells.
The first shell can hold a maximum of 2 electrons.
The second shell, where oxygen’s remaining electrons reside, can hold up to 8.
Ground State Electron Configuration of Oxygen
The electron configuration of oxygen in its ground state (lowest energy state) is written as 1s² 2s² 2p⁴
Here’s a breakdown of what this means:
1s²: The first two electrons are located in the 1s orbital, the lowest energy orbital closest to the nucleus.
2s²: The next two electrons occupy the 2s orbital, the second energy level’s s orbital.
2p⁴: The remaining four electrons are in the 2p orbital, which is part of the second energy level. This orbital can hold up to six electrons, but oxygen only needs four to complete its eight total electrons.
In visual terms, you can think of oxygen’s electron configuration like this: two electrons fill the inner shell (1s), and the remaining six electrons are distributed between the 2s and 2p orbitals in the second shell.
Why is Oxygen’s Electron Configuration Important?
The electron configuration of oxygen is vital for several reasons. It influences its chemical properties and interactions with other elements.
1. Bonding Behavior:
Oxygen’s electron configuration, specifically its six valence electrons (electrons in the outer shell), makes it highly reactive. It tends to gain two electrons to complete its valence shell, achieving a stable configuration similar to that of neon, a noble gas. This is why oxygen typically forms two bonds in molecules, such as water (H₂O) or oxygen gas (O₂).
2. Octet Rule:
The octet rule states that atoms are most stable when their outermost shell contains eight electrons. With six electrons in its second shell, oxygen needs two more to fulfill this rule. This drives its reactivity and tendency to form double bonds or accept two electrons in chemical reactions.
3. Oxygen’s Role in Compounds:
Oxygen’s electron configuration allows it to participate in covalent and ionic bonds. In covalent compounds, oxygen shares electrons to complete its octet, as seen in water. In ionic compounds like metal oxides, oxygen gains electrons from metals to form O²⁻ ions.
4. Reactivity and Combustion:
Oxygen’s electron configuration also explains its role in combustion and oxidation reactions. In these reactions, oxygen readily accepts electrons from other substances, fueling reactions that release energy, such as burning.
Orbital Diagram of Oxygen
To understand it better, we can visualize oxygen’s electron configuration using an orbital diagram. Each box represents an orbital in this diagram, and arrows represent electrons.
For oxygen:
1s orbital: Two electrons (↑↓)
2s orbital: Two electrons (↑↓)
2p orbitals: Four electrons (↑↓, ↑, ↑)
In the 2p orbitals, the first three electrons fill each p orbital singly (following Hund’s rule), and the fourth electron pairs up with one of the electrons in the orbitals. Google maps
Common Misconceptions about Oxygen’s Electron Configuration
There are a few misconceptions about electron configurations that are worth clearing up:
1. Some learners confuse the order in which orbitals are filled. Remember, the 2s orbital is always filled before the 2p orbitals.
2. Others might think oxygen always shares or gains electrons equally in all compounds. However, oxygen’s bonding behavior varies depending on the compound.
Learn more about Oxygen Electron Configuration in the main guide.
Conclusion
Oxygen’s electron configuration of 1s² 2s² 2p⁴ reveals much about its chemical nature. With six valence electrons, oxygen is highly reactive, striving to complete its octet by forming bonds with other atoms. Understanding this configuration is essential in fields ranging from chemistry to biology, as it governs oxygen’s behavior in compounds, reactions, and biological processes like respiration. Whether you’re studying basic chemistry or more advanced topics, knowing the electron configuration of oxygen provides a foundation for understanding its widespread role in the natural world.