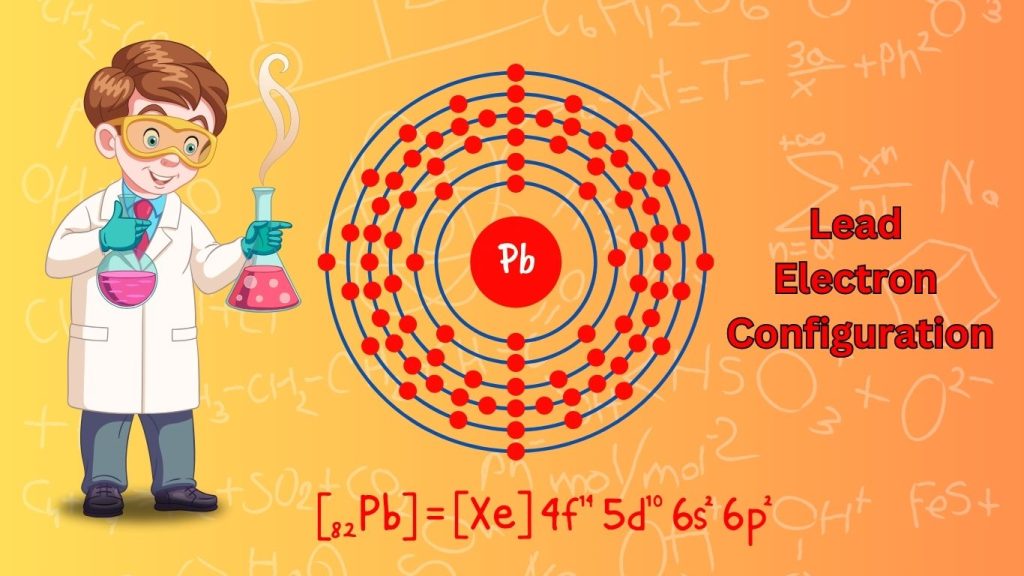
The Lead electron configuration is [Xe] 4f^14 5d^10 6s^2 6p^2. Lead is a chemical element with the symbol Pb and atomic number 82.
Lead is a soft, malleable heavy metal with a low melting point. Its electron configuration, which refers to the arrangement of electrons in its atoms, determines its chemical properties. The electron configuration of lead indicates that it has 82 electrons distributed across different energy levels and orbitals.
This configuration allows lead to exhibit characteristics that make it useful in various industrial applications, such as batteries, radiation shielding, and alloy manufacturing. Understanding lead’s electron configuration is vital in comprehending its chemical behavior and interactions with other elements.
Learn more about the Definition of Hydrogen Bond in the main guide.
What Is Electron Configuration
The electron configuration of an atom refers to the arrangement of its electrons in atomic orbitals. This arrangement provides essential information about the distribution of electrons in an atom, including the number of electrons in each energy level and sublevel. Understanding electron configuration is crucial for comprehending the chemical behavior of elements and their reactivity with other substances. Let’s delve into the definition and importance of electron configuration to gain a thorough understanding of this fundamental concept.
Definition
Electron configuration represents the distribution of electrons in an atom’s electron orbitals. It describes the arrangement of electrons in different energy levels and sublevels within an atom. The notation for electron configuration follows a specific format, incorporating numbers and letters to denote the configuration of electrons in an atom.
Importance
Understanding electron configuration is crucial in predicting an element’s chemical behavior and its interactions with other elements. The arrangement of electrons in an atom’s orbitals directly influences its chemical properties, such as reactivity, bonding, and the formation of compounds. By analyzing the electron configuration, scientists can gain valuable insights into an element’s behavior and its potential applications in various chemical processes and industries.
Bohr Model Of The Atom
The Bohr Model of the Atom is a fundamental concept in understanding the arrangement of electrons within an atom. Proposed by Danish physicist Niels Bohr in 1913, this model revolutionized our understanding of atomic structure. By introducing the idea of quantized energy levels, Bohr was able to explain why electrons don’t simply collapse into the nucleus.
Overview
The Bohr Model suggests that electrons orbit the nucleus in specific energy levels or shells. These energy levels are commonly labeled with the letters K, L, M, and so on, with the K shell closest to the nucleus. Each energy level can hold a specific number of electrons. The first energy level, K, can accommodate a maximum of 2 electrons, while the second level, L, can hold up to 8 electrons. Subsequent energy levels can hold even more electrons, following a specific pattern.
Electron Shell
The electron shell, also known as the electron cloud, is the region of space surrounding the nucleus where electrons are likely to be found. As mentioned earlier, these shells are labeled K, L, M, and so on, with each shell corresponding to a specific energy level. The electrons within a shell reside in orbitals, which are specific regions within the shell where electrons are most likely to be located.
It’s important to note that electrons in an atom occupy the lowest available energy levels before moving to higher levels. This means that the K shell is filled first before any electrons occupy the L shell. Similarly, the L shell is filled before the M shell, and so on. This electron configuration is vital in determining the chemical behavior and properties of elements.
For example, let’s consider the electron configuration of the element carbon. Carbon has an atomic number of 6, which means it has 6 electrons. Following the Bohr Model, the first 2 electrons would occupy the K shell, while the remaining 4 electrons would move to the L shell. This simple yet crucial understanding of electron configuration helps explain why carbon behaves the way it does in chemical reactions.
Summary
The Bohr Model of the Atom revolutionized our understanding of atomic structure by highlighting the importance of quantized energy levels and electron configuration. It introduced the concept of electron shells, which provide a framework for explaining the arrangement of electrons within an atom. Understanding electron configuration can give us insight into an element’s chemical behavior and properties.
Quantum Mechanical Model
The quantum mechanical model is a fundamental concept in chemistry that helps us understand the behavior of electrons within an atom. It combines the wave and particle properties of electrons to explain their behavior. In this blog post, we will explore the quantum mechanical model and its various aspects related to the electron configuration.
Wave-particle Duality
One of the key concepts in the quantum mechanical model is electrons’ wave-particle duality. According to this concept, electrons can exhibit characteristics of both waves and particles simultaneously. The idea, proposed by the famous physicist Louis de Broglie, revolutionized our understanding of the atomic world. It suggests that electrons, which were traditionally thought of as particles, can also display wave-like properties such as interference and diffraction.
An essential consequence of the wave-particle duality is that electrons do not have well-defined orbits like planets around the Sun. Instead, they exist in a state of probability known as an orbital. These orbitals are three-dimensional regions in space where the likelihood of finding an electron is high. Each orbital has a specific shape, which helps determine the electron’s energy and position within an atom.
Orbitals
Orbitals are characterized by a set of quantum numbers that provide information about an electron’s energy, size, and shape. There are four quantum numbers: the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (ml), and the spin quantum number (ms).
The principal quantum number (n) indicates the electron’s energy level. The value of n can be any positive integer, with higher values corresponding to higher energy levels. The azimuthal quantum number (l) defines the shape of the orbital and can have values ranging from 0 to (n-1). It determines the subshell to which the electron belongs, such as s, p, d, or f.
The magnetic quantum number (ml) describes the orbital orientation in space and can have values ranging from -l to +l. It helps distinguish between different orbitals within a subshell. Lastly, the spin quantum number (ms) determines the spin direction of the electron, which can be either up or down.
Quantum numbers play a crucial role in electron configuration. They help us understand the distribution of electrons among different orbitals within an atom. By following specific rules, such as the Aufbau principle, the Pauli exclusion principle, and Hund’s rule, we can determine the electron configuration of any atom. This information is vital for predicting an element’s chemical behavior and understanding its properties.
Aufbau Principle
In the science of chemistry, the Aufbau principle is a crucial concept that helps us understand the electron configuration of atoms. This principle outlines the order in which electrons occupy orbitals in an atom based on their energy levels. Understanding the Aufbau principle is essential for grasping the arrangement of electrons in an atom, which is fundamental to comprehending the chemical properties and behavior of elements.
Explanation
The Aufbau principle states that electrons first fill the lowest energy orbitals before occupying higher energy levels. This means that electrons will first fill the 1s orbital, followed by the 2s orbital, and then the 2p orbital, and so on, according to the increasing order of energy levels.
Order Of Filling
The order of filling orbitals in the Aufbau principle follows a specific pattern based on the increasing energy levels. The table below illustrates the order of filling for the first few electron orbitals:
| Energy Level | Sublevel | Electron Configuration |
|---|---|---|
| 1 | 1s | 1s2 |
| 2 | 2s | 2s2 |
| 2 | 2p | 2p6 |
Pauli Exclusion Principle
The Pauli Exclusion Principle dictates the electron configuration of lead, ensuring that no two electrons can occupy the same quantum state. This principle plays a crucial role in understanding the arrangement of electrons within an atom.
Explanation
The Pauli Exclusion Principle is a fundamental concept in quantum mechanics. It states that no two electrons in an atom can have the same set of quantum numbers. In simpler terms, this means that each electron must have a unique set of properties, such as its energy level, angular momentum, and spin.
Spin Of Electrons
One property that differentiates electrons is their spin. The spin of an electron is denoted by the quantum number “s” and can have two possible values: +1/2 and -1/2. This means that electrons can be thought of as spinning clockwise or counterclockwise on their own axis.
- Electrons with a spin of +1/2 are often referred to as “spin-up” electrons.
- On the other hand, electrons with a spin of -1/2 are known as “spin down” electrons.
The spin of an electron plays a crucial role in determining its behavior and its interactions with other particles. It influences how electrons occupy different energy levels in an atom, ensuring that no two electrons occupy the same energy level with the same set of quantum numbers.
It is worth noting that an electron’s spin is not an actual spinning motion in the classical sense. Rather, it is an intrinsic property of the electron that cannot be visualized in terms of everyday objects.
In summary, the Pauli Exclusion Principle governs the behavior of electrons in atoms, ensuring that each electron occupies a unique set of quantum numbers. The spin of electrons is one such property that distinguishes them from one another. By obeying these principles, electrons contribute to the stability and structure of atoms, influencing various chemical and physical properties.
Hund’s Rule
Hund’s rule governs the arrangement of electrons in lead’s electron configuration, ensuring that electrons fill orbitals singly before pairing up. This rule is crucial in understanding lead’s chemical properties and behavior.
Explanation
In chemistry, Hund’s Rule is a fundamental principle that helps us understand the electron configuration of atoms and their stability. It states that when filling orbitals of the same energy level, such as the p, d, or f orbitals, electrons prefer to occupy separate orbitals within the same sublevel rather than pairing up in a single orbital. This rule applies to atoms in their ground or lowest energy states. Let’s dive deeper into Hund’s Rule and understand why it is important to understand the electron configuration of atoms.
Stability Of Half-filled And Fully-filled Orbitals
One of Hund’s Rule’s key implications is the stability of half-filled and fully-filled orbitals. The atom is more stable when an orbital is half-filled or fully filled than other configurations. This stability arises from the mutual repulsion between electrons occupying the same orbital, known as electron-electron repulsion.
To understand this better, let’s take an example of chromium (Cr) with an atomic number of 24. Chromium’s electron configuration in its ground state is 1s2 2s2 2p6 3s2 3p6 4s1 3d5. Here, we can observe that the 3d sublevel is only half-filled, with 5 electrons occupying 5 separate orbitals. The half-filled nature of the 3d sublevel contributes to the higher stability of chromium.
Similarly, let’s consider the example of argon (Ar) with an atomic number of 18. Its electron configuration is 1s2 2s2 2p6 3s2 3p6. Argon’s electron configuration corresponds to a fully filled third energy level. The stability of argon primarily arises from the complete filling of all available orbitals in the 3s and 3p sub-levels.
Moreover, the stability associated with half-filled and fully-filled orbitals plays a significant role in explaining the behavior of transition metals and rare earth elements. These elements often exhibit unique chemical properties due to their partially filled d and f orbitals, contributing to their reactivity, magnetic properties, and ability to form colorful compounds.
In conclusion, Hund’s Rule is a crucial concept in understanding the electron configuration of atoms. It explains why electrons prefer to occupy separate orbitals within the same sublevel rather than pair up. Additionally, the stability associated with half-filled and fully-filled orbitals is a direct consequence of this rule, contributing to the unique properties observed in various elements.
Writing Electron Configurations
Writing electron configurations is the process of representing the distribution of electrons within an atom’s orbitals. This notation is essential in understanding the behavior and properties of elements. It provides a concise way to depict the location of electrons in an atom, which is crucial in chemistry and physics. Understanding how to write electron configurations is paramount to comprehending an element’s chemical behavior and reactivity.
Notation
The notation for writing electron configurations follows a specific format. It begins with the principal quantum number, followed by the subshell denoted by the letter (s, p, d, or f) and the superscript indicating the number of electrons in that subshell. For instance, the electron configuration for carbon is written as 1s2 2s2 2p2.
Examples
To illustrate this further, let’s consider the electron configuration for oxygen, which is 1s2 2s2 2p4. This represents the arrangement of eight electrons within an oxygen atom’s energy levels and sublevels.
Exceptions To Electron Configuration
When discussing electron configuration, it is important to note that there are exceptions to the usual patterns seen in atoms. These exceptions occur primarily in transition metals, lanthanides, and actinides. Understanding these exceptions can provide valuable insights into the behavior and properties of these elements.
Transition Metals
Transition metals in the periodic table’s middle section exhibit variations in their electron configuration due to the presence of d orbitals. Unlike other elements, transition metals can have multiple oxidation states, which can be attributed to the varying numbers of electrons in their outermost s and d orbitals. For example, in the element chromium (Cr), the expected electron configuration would be [Ar] 3d4 4s2. However, the actual configuration is [Ar] 3d5 4s1. This occurs because having one electron in the 4s orbital increases stability for the entire atom. This exception is known as the “half-filled” d orbital stability.
Another example of an exception in transition metals is copper (Cu). The expected electron configuration would be [Ar] 3d9 4s2, but the actual configuration is [Ar] 3d10 4s1. Like chromium, this configuration provides increased stability due to the completely filled 3d orbital. This phenomenon is known as the “fully-filled” d orbital stability.
Lanthanides And Actinides
Lanthanides and actinides, commonly referred to as rare earth elements, also exhibit exceptions to electron configuration due to their unique electron arrangement. These elements have partially filled 4f and 5f orbitals, leading to deviations from the expected patterns.
For instance, the element gadolinium (Gd) deviates from the expected electron configuration of [Xe] 6s2 5d1 4f7 and instead has a configuration of [Xe] 6s2 4f7 5d1. This exception occurs because filling the 4f orbital before the 5d orbital results in greater stability for the atom.
Similarly, the element uranium (U) deviates from the expected configuration of [Rn] 7s2 5f3 6d1 and has a configuration of [Rn] 7s2 5f3 6d1. This exception arises due to the stability gained by filling the 5f orbital before the 6d orbital.
In Summary
Transition metals, lanthanides, and actinides exhibit exceptions to electron configuration. These exceptions occur due to the presence of d and f orbitals and their influence on stability. Understanding these exceptions can illuminate these elements’ unique properties and behavior.
Significance Of Electron Configuration
The electron configuration of lead holds significant value as it determines the arrangement of electrons in its energy levels. This configuration plays a crucial role in understanding lead’s chemical properties and reactivity, shedding light on its behavior in various chemical reactions and bonding with other elements.
Understanding an atom’s electron configuration is crucial to comprehending its chemical behavior. The arrangement of electrons in the electron shells plays a vital role in determining an element’s stability, reactivity, and various properties. By analyzing the electron configuration, scientists can predict the likelihood of an atom bonding with other atoms and forming compounds. Additionally, the electron configuration provides valuable insights into the periodic trends of elements, helping us classify and understand elements based on their chemical reactivity and properties.
Chemical Reactivity
Chemical reactivity refers to how easily an element can participate in chemical reactions and form compounds with other elements. The electron configuration directly influences an element’s reactivity. Elements with incomplete or partially filled outer electron shells tend to be highly reactive as they seek stability by either gaining, losing, or sharing electrons with other atoms. This behavior is exemplified by the alkali metals, such as sodium and potassium, which have a single valence electron residing in their outermost shell. These metals tend to lose this electron, resulting in their high reactivity. On the other hand, elements with filled outer electron shells, like noble gases, are chemically unreactive due to their electron configuration, making them stable and non-reactive.
Periodic Trends
Periodic trends refer to the patterns and variations in the properties of elements as one moves across or down the periodic table. These trends can be understood and explained by analyzing the electron configuration. The electron configuration determines an element’s position in the periodic table, allowing us to identify trends in properties such as atomic radius, ionization energy, electronegativity, and metallic character. For example, as one progresses from left to right across a period, the atomic radius generally decreases due to the increasing number of protons in the nucleus, resulting in stronger attractive forces on the electrons. This trend is a direct consequence of the electron configuration and highlights the significance of understanding electron arrangements in predicting and explaining periodic trends. Google Maps.
Applications
The lead electron configuration has various applications in the field of chemistry and materials science. It plays a crucial role in predicting element properties and understanding chemical bonding.
Prediction Of Element Properties
The electron configuration of lead, which is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2, helps in predicting its physical and chemical properties. This information is valuable in determining its reactivity, melting point, boiling point, and other essential characteristics for various industrial processes.
Understanding Chemical Bonding
Lead’s electron configuration aids in understanding how lead forms chemical bonds with other elements. It enables scientists to comprehend the nature of the bonds and the stability of lead compounds, which is vital for developing new materials and pharmaceuticals.
Frequently Asked Questions For Lead Electron Configuration
How Do You Write The Electron Configuration For Lead?
The electron configuration for lead is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2.
Which Element Has The Electron Configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d6?
The element with the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d6 is Chromium.
What Element Has An Electron Configuration Of 1s 2 2s 2 2p 6 3s 2 3p 3?
The element with this electron configuration is phosphorus. It has 15 electrons and is found in the third period of the periodic table.
What Is The Electron Configuration Of Br?
The electron configuration of Br is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5.
Conclusion
Understanding the electron configuration of lead plays a crucial role in comprehending its chemical properties and behavior. This knowledge can aid in various scientific and industrial applications. By delving into the intricacies of lead’s electron arrangement, researchers and professionals can unlock new potentials for utilizing this element in diverse fields.
