Xenon has eight valence electrons. It is a noble gas with a complete outer shell.
Xenon is a chemical element with the symbol Xe and atomic number 54. It belongs to the noble gases group in the periodic table. Noble gases are known for their chemical inertness due to a full valence electron shell. This makes xenon stable and less reactive compared to other elements.
Xenon is used in various applications, including lighting, anaesthesia, and ion propulsion systems. Its unique properties make it valuable in both industrial and scientific fields. Understanding xenon’s valence electrons helps comprehend its chemical behaviour and interactions.
Introduction To Xenon
Xenon is a fascinating element on the periodic table. Its symbol is Xe, and its atomic number is 54. Xenon belongs to the noble gases group and is known for its unique properties.
In this blog post, we will explore the number of valence electrons xenon has. Understanding xenon’s properties helps us appreciate its role in various applications.
Noble Gas Family
Xenon is part of the noble gas family, which is known for its stability and lack of reactivity. Other elements in this group include helium, neon, argon, krypton, and radon.
These gases have a full valence shell, making them very stable. This stability is due to having eight electrons in their outer shell.
| Element | Symbol | Atomic Number |
|---|---|---|
| Helium | He | 2 |
| Neon | Ne | 10 |
| Argon | Ar | 18 |
| Krypton | Kr | 36 |
| Xenon | Xe | 54 |
| Radon | Rn | 86 |
Unique Properties
Xenon has some unique properties that set it apart. It is colourless, dense, and odourless. Unlike other gases, xenon can form compounds, although rarely.
It is used in lighting, medical imaging, and space travel. Its ability to emit bright light makes it ideal for flash lamps and arc lamps.
Xenon is used in medical imaging for anaesthesia and diagnostic imaging. Its properties make it safe and effective for these uses.
Due to its high atomic weight and ionization potential, xenon is used as a propellant in ion thrusters in space travel.
Electron Configuration Basics
Understanding an element’s electron configuration helps us determine its chemical properties. This includes knowing how many valence electrons it has. Valence electrons are crucial for bonding and reactions.
Atomic Structure
Every element has a unique atomic structure. This structure consists of protons, neutrons, and electrons.
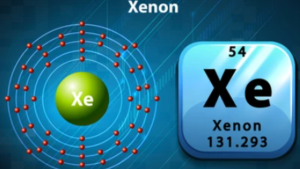
The element is defined by the number of protons in the nucleus. Xenon has 54 protons. Electrons orbit around the nucleus in regions called shells.
Electron Shells
Electrons occupy different shells or energy levels around the nucleus. Each shell can hold a specific number of electrons.
Here is a table showing the maximum number of electrons each shell can hold:
| Shell | Maximum Electrons |
|---|---|
| 1 (K) | 2 |
| 2 (L) | 8 |
| 3 (M) | 18 |
| 4 (N) | 32 |
Xenon has a total of 54 electrons. These electrons fill the shells in a specific order. The electron configuration for Xenon is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶
In this configuration, the numbers represent energy levels, and the letters denote sub-levels. The superscripts show the number of electrons in each sublevel.
The outermost shell of Xenon is the fifth shell, which has eight valence electrons. These valence electrons play a key role in chemical reactions.
Valence Electrons Explained
Understanding valence electrons is key in chemistry. These electrons determine how atoms interact and bond. Let’s explore their significance further.
Definition And Importance
Valence electrons are the outermost electrons of an atom. They are in the atom’s highest energy level. These electrons are crucial because they participate in chemical bonding.
Atoms bond to achieve a full outer shell. This usually means having eight valence electrons, known as the octet rule. Atoms with full outer shells are more stable.
Role In Chemical Reactions
Valence electrons play a vital role in chemical reactions. Atoms gain, lose, or share these electrons to form bonds, which create molecules and compounds.
For example, xenon has eight valence electrons, which makes it stable and less reactive. However, under certain conditions, xenon can still form compounds by sharing electrons.
Xenon’s Electron Configuration
Xenon is a noble gas with a unique electron configuration. Understanding this configuration helps in grasping its chemical properties. Let’s examine Xenon’s atomic number, shells, and electron distribution.
Atomic Number And Shells
Xenon has an atomic number of 54. The atomic number is the number of protons in an atom. Xenon’s electrons are arranged in different shells around the nucleus.
The shells are like layers where electrons orbit. Each shell can hold a specific number of electrons. Xenon’s electron shells are filled in a specific order.
| Shell | Maximum Electrons |
|---|---|
| 1st Shell | 2 |
| 2nd Shell | 8 |
| 3rd Shell | 18 |
| 4th Shell | 18 |
| 5th Shell | 8 |
Detailed Electron Distribution
Let’s look at Xenon’s electron distribution in detail. Xenon’s electrons fill the shells in a specific way:
- 1st shell: 2 electrons
- 2nd shell: 8 electrons
- 3rd shell: 18 electrons
- 4th shell: 18 electrons
- 5th shell: 8 electrons
So, the electron configuration of Xenon is written as 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6.
Valence electrons are the electrons in the outermost shell. Xenon has 8 valence electrons. These electrons determine how Xenon interacts with other elements.
Determining Xenon’s Valence Electrons
Understanding xenon’s valence electrons helps comprehend its chemical properties. Xenon is a noble gas found in Group 18 of the periodic table. Knowing the number of valence electrons is crucial for studying chemical reactions and bonding behaviour. Let’s delve into the details.
Outer Shell Electrons
Xenon’s atomic number is 54, indicating it has 54 electrons. These electrons are arranged in different energy levels or shells. The valence electrons are in the outermost shell.
The electron configuration of xenon is: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6. Here, the outermost shell is the fifth shell.
In the fifth shell, xenon has eight electrons: 5s2 4d10 5p6. This makes xenon very stable and unreactive.
Stable Configuration
Xenon has a full outer shell with eight valence electrons, which is called an octet configuration. This configuration is very stable.
The stable configuration is why xenon rarely forms compounds. Most noble gases exhibit similar stability due to their full outer shells.
Here’s a table summarizing xenon’s electron configuration:
| Shell | Number of Electrons |
|---|---|
| 1st Shell | 2 |
| 2nd Shell | 8 |
| 3rd Shell | 18 |
| 4th Shell | 18 |
| 5th Shell | 8 |
In summary, xenon has eight valence electrons in its outermost shell. This contributes to its stability and lack of reactivity.
Chemical Behavior Of Xenon
Xenon is a noble gas with eight valence electrons. Its chemical behaviour is unique among the elements. Below, we explore the inert nature and rare compounds of Xenon.
Inert Nature
Xenon is a noble gas in the periodic table. Noble gases are known for their inert nature, which means they do not react easily with other elements. Xenon has a full valence shell with eight electrons, which makes It very stable.
Because of its stability, Xenon does not form compounds easily. It requires high energy or special conditions to react. This inertness is a key characteristic of Xenon.
Rare Compounds
Although Xenon is inert, it can form some rare compounds under special conditions. These compounds usually involve strong oxidizing agents.
Some known compounds of Xenon include:
- Xenon hexafluoroplatinate (XePtF6)
- Xenon difluoride (XeF2)
- Xenon tetrafluoride (XeF4)
- Xenon hexafluoride (XeF6)
These compounds show that Xenon can be reactive under the right conditions. Chemists use these compounds in various research and industrial applications.
| Compound | Formula | Uses |
|---|---|---|
| Xenon hexafluoroplatinate | XePtF6 | Research in noble gas chemistry |
| Xenon difluoride | XeF2 | The oxidizing agent in organic synthesis |
| Xenon tetrafluoride | XeF4 | Fluorinating agent in chemical reactions |
| Xenon hexafluoride | XeF6 | Strong fluorinating agent |
Applications Of Xenon
Xenon, a noble gas, has many unique applications. Its properties make it useful in various fields. Let’s explore some of the fascinating uses of xenon.
Lighting And Lasers
Xenon is widely used in lighting. Xenon arc lamps produce bright, white light. These lamps are common in projectors and searchlights. Xenon flash lamps are essential in photography. They help capture high-speed images.
In the world of lasers, xenon has a special place. Xenon ion lasers emit blue and green light, which is useful in scientific research. Excimer lasers use xenon to produce ultraviolet light, which is vital in eye surgery and semiconductor manufacturing. Google maps
Medical Uses
Xenon has significant medical applications. It is an effective anesthetic. Xenon gas is used during surgeries because it has a quick recovery time and minimal side effects.
Xenon is also valuable in imaging. Xenon-enhanced MRI provides clearer images of the brain, which helps diagnose various conditions. Xenon-133 is a radioactive isotope used in lung imaging to detect breathing issues.
| Application | Use |
|---|---|
| Lighting | Projectors, Searchlights, Photography |
| Lasers | Scientific Research, Eye Surgery, Semiconductor Manufacturing |
| Medical | Anesthesia, Brain Imaging, Lung Imaging |
Frequently Asked Questions
What Are Valence Electrons In Xenon?
Valence electrons are the outermost electrons of an atom. In xenon, these electrons determine its chemical properties and reactions.
How Many Valence Electrons Does Xenon Have?
Xenon has eight valence electrons. This makes it stable and less likely to react with other elements.
Why Is Xenon Stable With Eight Valence Electrons?
Eight valence electrons provide a full outer shell. This makes xenon chemically inert and stable under normal conditions.
Can Xenon Form Compounds With Other Elements?
Yes, xenon can form compounds. It can react under specific conditions to form xenon fluorides and oxides despite being inert.
Conclusion
Understanding xenon’s valence electrons is crucial for chemistry enthusiasts. Xenon has eight valence electrons, giving it unique chemical properties. This knowledge aids in grasping its reactivity and bonding behaviour. Stay curious and keep exploring the fascinating world of elements and their electrons.
Your chemistry knowledge will only grow richer.
