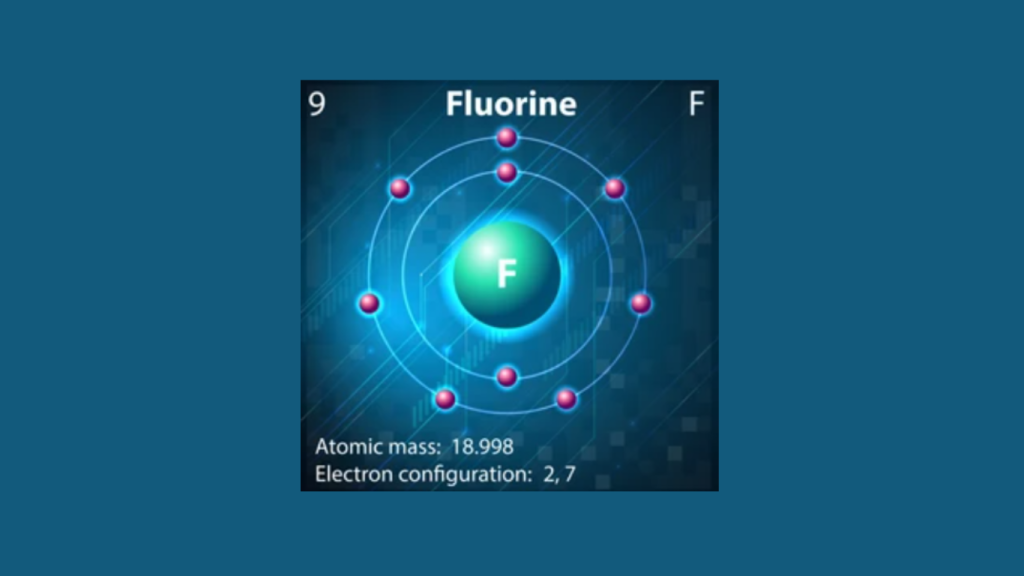
The Fluorine Electron Configuration is 1s^2 2s^2 2p^5. Fluorine has a total of 9 electrons, with 2 in the 1s orbital, 2 in the 2s orbital, and 5 in the 2p orbital.
Its electron configuration reflects its atomic structure and the arrangement of electrons in its energy levels. Fluorine, with the chemical symbol F, is a highly reactive and abundant element in the halogen group of the periodic table. It is a pale yellow gas at room temperature with a strong affinity for electrons, readily forming compounds with other elements.
Fluorine’s electron configuration is crucial in understanding its chemical properties and reactivity.
What Is Fluorine?
Fluorine is a highly reactive, light yellow-green gas from the halogen group, located in the periodic table’s group 17. It is the most electronegative element and has the smallest atomic radius among the halogens. Let’s take a closer look at the chemical and physical properties of fluorine.
Chemical Properties Of Fluorine
Fluorine is known for its powerful oxidizing and corrosive properties. It readily forms compounds with almost all other elements except helium, neon, and argon. Being highly electronegative, it attracts electrons, making it extremely reactive and capable of forming strong bonds.
Physical Properties Of Fluorine
Fluorine is a pale yellow-green gas at room temperature and pressure, with a characteristic pungent odor. It liquefies at -188.13°C and solidifies at -219.67°C. Due to its high electronegativity and small size, it exists as a diatomic molecule (F2). This element is essential in various industrial processes and plays a crucial role in the manufacturing of numerous products, such as refrigerants, plastics, and pharmaceuticals.
Electron Configuration Basics
Discovering the basics of electron configuration, specifically focusing on the fluorine element, provides insights into its arrangement of electrons. Gain a deeper understanding of fluorine’s electron configuration for a comprehensive grasp of its chemical properties.
Overview Of Electron Configuration
Welcome to the exciting world of electron configuration! In this section, we will dive into the fundamentals of electron configuration, helping you understand the arrangement of electrons in an atom. By grasping this concept, you’ll gain valuable insights into the behavior and properties of elements.
Importance Of Electron Configuration
Understanding electron configuration is crucial as it provides a roadmap to comprehending the chemical behavior of elements. By knowing the arrangement of electrons in an atom’s energy levels and sublevels, scientists can predict how an element will interact with other substances, its reactivity, and even its physical properties. This knowledge becomes especially useful when exploring the wonderful world of fluorine.
Why is electron configuration so important when studying fluorine specifically? Well, fluorine’s remarkable chemical reactivity and unique properties can be attributed to its electron configuration.
Fluorine (symbol: F) belongs to the halogen family and is located in group 17 of the periodic table. It has atomic number 9, meaning it has 9 electrons. The electron configuration of fluorine is 1s2 2s2 2p5. Let’s break that down:
- The first energy level (1s) contains 2 electrons.
- The second energy level (2s) contains 2 electrons as well.
- The remainder of the electrons, 5 to be precise, fill the 2p energy sublevel.
The electron configuration of fluorine reveals that it has a nearly complete outer electron shell with 7 valence electrons. This makes it highly reactive and eager to gain an additional electron to achieve a stable octet configuration.
This configuration gives fluorine its strong oxidizing properties, allowing it to readily form compounds with other elements. It also explains why fluorine is a powerful diatomic molecule, widely used in industries ranging from pharmaceuticals to manufacturing.
Now that you appreciate the importance of electron configuration and its impact on understanding elements let’s explore more fascinating aspects of fluorine and its electron behavior.
Atomic Structure Of Fluorine
Understanding the atomic structure of fluorine is key to comprehending its behavior and properties. In this section, we will delve into the various components of a fluorine atom, including the number of protons, electrons, and neutrons.
Number Of Protons And Electrons In A Fluorine Atom
A fluorine atom consists of 9 protons and 9 electrons.
Neutrons In A Fluorine Atom
While the number of protons and electrons in a fluorine atom is the same, the number of neutrons may vary. Typically, a fluorine atom contains 10 neutrons; however, there are also fluorine isotopes that contain 8 or 9 neutrons.
The atomic structure of fluorine can be better understood through a table:
| Component | Number |
|---|---|
| Protons | 9 |
| Electrons | 9 |
| Neutrons | 10 |
By knowing the number of protons, electrons, and neutrons in a fluorine atom, scientists can better understand its chemical behavior and its position in the periodic table. Fluorine’s atomic structure contributes to its valence electron configuration and its ability to form bonds with other elements.
Now that we have explored fluorine’s atomic structure let’s explore its valence electron configuration in the next section.
The Aufbau Principle
The Aufbau Principle:
The Aufbau principle is a fundamental concept in chemistry that explains the arrangement of electrons in atoms. It is based on the idea that electrons fill atomic orbitals in a specific order, with lower-energy orbitals filling before higher-energy ones. The principle helps determine an atom’s electron configuration, which is essential for understanding its chemical properties.
Explanation Of The Aufbau Principle
The Aufbau principle is rooted in the idea that electrons occupy the lowest available energy levels before filling higher ones. This principle is crucial in understanding how electrons are arranged within an atom. According to the Aufbau principle, the filling of electron orbitals follows a specific pattern, allowing us to predict the electron configuration of any given element.
How The Aufbau Principle Determines Electron Configuration
Using the Aufbau principle, we can determine the electron configuration of an element by following a specific order in which the orbitals are filled. This ensures that each electron is placed in the lowest available energy level before moving on to higher ones. Adhering to this principle allows us to accurately determine the arrangement of electrons in an atom, which is vital for understanding its chemical behavior and reactivity.
Fluorine’s Electron Configuration
Fluorine’s electron configuration is a fascinating topic in chemistry. Understanding how its electrons are arranged can provide valuable insights into this element’s chemical behavior and properties.
Ground State Electron Configuration Of Fluorine
The ground state electron configuration of fluorine is [He] 2s2 2p5. Let’s break down this electron configuration:
- [He] represents the electron configuration of the noble gas helium, used as a shorthand to denote the first two energy levels, which are already filled with electrons.
- The 2s orbital can hold a maximum of 2 electrons. In the case of fluorine, we find that there are 2 electrons occupying this orbital.
- The 2p orbital, which consists of three suborbital (2px, 2py, and 2pz), can hold a total of 6 electrons. In the case of fluorine, we find that there are 5 electrons occupying the 2p orbital.
This arrangement of electrons in fluorine’s ground state gives it a total of 9 electrons. With 7 valence electrons, fluorine gains one more electron to achieve a stable electron configuration, making it a highly reactive and electronegative element.
Excited State Electron Configuration Of Fluorine
When fluorine is excited, its electrons can be promoted to higher energy levels, resulting in an excited state electron configuration. For example, the excited state electron configuration of fluorine can be represented as [He] 2s2 2p4 3s1.
In this excited state, one electron from the 2p orbital moves to the 3s orbital. This arrangement is only temporary and highly unstable. Once the energy source is removed, the electrons will return to their ground state configuration.
By understanding fluorine’s electron configurations in both its ground state and excited state, scientists can gain a deeper understanding of its chemical reactivity and ability to form compounds with other elements.
Understanding Energy Levels
Understanding energy levels is crucial in comprehending the concept of the fluorine electron configuration. Energy levels refer to the different states that an electron can occupy within an atom. Each energy level represents a specific amount of energy that an electron possesses. In this section, we will delve deeper into the definition of energy levels and how they relate to electron configuration.
Definition Of Energy Levels
Energy levels can be considered different orbits or shells around an atom’s nucleus where electrons can reside. These energy levels are quantized, meaning that they only exist at specific, discrete energy values. Think of it like a ladder; each rung represents an energy level that an electron can occupy. The lowest energy level, also known as the ground state, is closest to the nucleus, while the higher energy levels are further away.
How Energy Levels Relate To Electron Configuration
The arrangement of electrons in an atom’s energy levels is known as electron configuration. This configuration determines an element’s chemical properties. Each energy level has a maximum number of electrons it can hold, with the innermost level holding only two electrons and the subsequent levels accommodating more. The distribution of electrons across these energy levels follows a specific pattern, with lower energy levels filled before higher ones.
For fluorine, which has an atomic number of 9, the electron configuration is 1s2 2s2 2p5. This means that the first energy level (1s) is filled with two electrons, the second energy level (2s) with two electrons, and the remaining five electrons are accommodated in the 2p energy level. Understanding the electron configuration of fluorine helps us decipher its reactivity and chemical behavior and provides insights into how it forms compounds.
Electron Spin And The Pauli Exclusion Principle
Understanding the electron spin and the Pauli exclusion principle is crucial when discussing the electron configuration of fluorine. Let’s dive into an explanation of electron spin and how the Pauli exclusion principle impacts the electron configuration of this element.
Explanation Of Electron Spin
Electron spin is a fundamental property of electrons, representing their intrinsic angular momentum. This property can be described as spin-up and spin-down, denoted by the quantum numbers +1/2 and -1/2, respectively. The concept of electron spin is an essential component in understanding the behavior and arrangement of electrons within an atom.
How The Pauli Exclusion Principle Affects Electron Configuration
The Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers. This principle directly affects the electron configuration by dictating the arrangement of electrons into different orbitals within an atom’s electron shells. As a result, it influences the overall stability and properties of the atom.
The Role Of Valence Electrons
Valence electrons play a crucial role in determining the characteristics of elements. Fluorine’s electron configuration is 2-7, giving it a full outer shell and making it highly reactive. This configuration contributes to its strong electronegativity and its ability to form compounds with other elements.
Valence electrons play a crucial role in an element’s chemical behavior, dictating its reactivity and forming the basis of bonding. Understanding the concept of valence electrons is essential in comprehending the electron configuration of elements like fluorine and how they interact with other elements. In this article, we will explore the definition of valence electrons and discuss their importance in chemical reactions.
Definition Of Valence Electrons
Valence electrons are located in an atom’s outermost energy level, or valence shell. They are involved in forming chemical bonds and determining the element’s chemical properties. The number of valence electrons an atom possesses is crucial in predicting its reactivity and its ability to form compounds with other atoms.
Importance Of Valence Electrons In Chemical Reactions
Valence electrons determine how an element will behave during a chemical reaction. The number of valence electrons influences an atom’s ability to gain, lose, or share electrons to achieve a more stable electron configuration, typically the electron configuration of a noble gas.
The reactivity of an element can be determined by the number of valence electrons it possesses. Elements with only a few valence electrons tend to react highly as they seek to gain or lose electrons to reach a stable configuration. On the other hand, elements with a full valence shell, such as noble gases, have low reactivity because their electron configurations are already stable.
Valence electrons are responsible for the formation of chemical bonds between atoms. Atoms can either share valence electrons through covalent bonds or transfer valence electrons to form ionic bonds. The interaction of valence electrons between atoms allows for creating compounds and transforming pure elements into new substances.
In summary, valence electrons are essential in understanding the chemical behavior of elements. Their role in chemical reactions determines the reactivity and bonding capabilities of atoms. By gaining insight into the number and behavior of valence electrons, we can better understand the properties and reactions of elements like fluorine and their impact on the world around us.
Comparison With Other Elements
When we compare the electron configuration of fluorine with other elements, we can better understand its properties and behavior. Let’s explore how fluorine’s electron configuration compares both within its group and with elements in other groups.
Comparison Of Fluorine’s Electron Configuration With Other Elements In Its Group
Fluorine belongs to Group 17, also known as the halogens. Its electron configuration is 1s2 2s2 2p5, meaning it has nine electrons. Some interesting patterns emerge when we compare this to other elements in the same group, such as chlorine, bromine, iodine, and astatine.
All of these elements have seven electrons in the outermost energy level (the second energy level), making them highly reactive. This is due to the fact that they only require one additional electron to achieve a stable octet configuration. However, fluorine has the lowest atomic radius among the halogens, making it the most electronegative element in the group. This means that fluorine strongly tends to attract electrons, making it the most reactive of all the halogens.
Comparison With Elements In Other Groups
Beyond its own group, fluorine’s electron configuration places it among the nonmetals in the periodic table. When we compare fluorine with elements in different groups, such as oxygen in Group 16 and nitrogen in Group 15, some interesting trends become apparent.
Fluorine, with its outer electron configuration of 2s2 2p5, has a similar electron configuration to oxygen (1s2 2s2 2p4), which also has a strong tendency to attract electrons. This is one reason why both fluorine and oxygen form stable compounds with each other, such as hydrogen fluoride (HF) and oxygen difluoride (OF2).
On the other hand, when we compare fluorine with nitrogen (1s2 2s2 2p3), we see that nitrogen has one less electron in its outer energy level. This difference in electron configuration greatly affects their reactivity. While fluorine readily accepts an electron to achieve a stable octet configuration, nitrogen prefers to share electrons with other elements. This difference in reactivity is why fluorine and nitrogen have different chemical properties.
Chemical Reactivity Of Fluorine
Fluorine’s chemical reactivity is influenced by its electron configuration, which consists of 9 protons and 9 electrons arranged in the 1s22s22p5 configuration. This unique configuration makes fluorine the most electronegative element, with a strong tendency to attract electrons, leading to its highly reactive nature.
How The Electron Configuration Of Fluorine Influences Its Reactivity
The electron configuration of fluorine, marked by the presence of seven valence electrons, creates a strong desire to fill its outer shell for stability. This leads to its remarkable reactivity, as fluorine aggressively seeks to acquire an additional electron to achieve an octet, resulting in strong bonds with other elements.
Examples Of Chemical Reactions Involving Fluorine
Fluorine participates in various chemical reactions, demonstrating its reactivity. Some prominent examples include:
- Formation of hydrogen fluoride through the reaction of fluorine with hydrogen gas.
- Fluorination of organic compounds, where fluorine replaces hydrogen atoms in various molecules.
- Reacting with metals such as aluminum leads to the formation of aluminum fluoride.
Applications Of Fluorine
Fluorine, denoted by the symbol F, is an essential chemical element with many applications in various industries and medical and dental fields. Its unique properties and reactivity make it a valuable element for numerous processes and products. In this section, we will explore fluorine’s different applications and how it contributes to various sectors.
Industrial Uses Of Fluorine
In the industrial sector, fluorine finds extensive applications due to its reactivity and ability to form strong chemical bonds. Some of the key industrial uses of fluorine include:
- Manufacturing of Teflon: Fluorine is crucial in producing Teflon, a well-known nonstick coating material. With its high heat resistance and low friction properties, Teflon is widely used in cookware, electrical insulation, and various industrial applications.
- Fluorine gas production: Fluorine gas is used in several industrial processes such as uranium processing, petroleum refining, and the production of chemicals like sulfur hexafluoride (SF6) and Freon. These chemicals have various applications, including electrical insulation, refrigeration, and solvents.
- Medical gas: Fluorine gas is used as an anesthetic during surgical procedures, primarily as a component of volatile anesthetic agents like halothane. It provides safe and effective sedation for patients undergoing surgery.
- Electronics manufacturing: Fluorine-based plasma etching is commonly employed in the semiconductor industry for microchip fabrication. It helps remove unwanted layers and create precise patterns on silicon wafers, enabling the production of smaller and more efficient electronic devices.
Medical And Dental Applications Of Fluorine
Fluorine plays a crucial role in medical and dental applications, contributing to the prevention and treatment of various conditions. The use of fluorine in medicine and dentistry includes:
- Water fluoridation: Fluoride compounds are added to public water supplies, promoting dental health by preventing tooth decay. Fluoridation has been instrumental in reducing dental caries worldwide, especially in communities with limited access to dental care.
- Fluoride toothpaste and mouthwash: Fluorine compounds like sodium fluoride are commonly used in oral hygiene products such as toothpaste and mouthwash. These products help strengthen tooth enamel, prevent cavities, and maintain overall oral health.
- Fluoride treatments: Dentists often apply fluoride treatments to the teeth of patients at risk of dental decay. These treatments help remineralize teeth, making them more resistant to acid attacks and reducing the likelihood of cavities.
- Fluorine-based medical imaging: Positron emission tomography (PET) scans and fluorodeoxyglucose (FDG) imaging techniques utilize fluorine-18, a radioactive isotope of fluorine. These imaging methods are employed to detect and diagnose various diseases, including cancer and heart conditions. Google maps
Frequently Asked Questions On Fluorine Electron Configuration
How Do You Write The Electron Configuration For Fluorine?
The electron configuration for Fluorine is 1s2 2s2 2p5.
Which Element Is Represented By The Electronic Configuration 1s2 2s2 2p6 3s2 3p6 4s1?
The element represented by the electronic configuration 1s2 2s2 2p6 3s2 3p6 4s1 is potassium.
How Does Fluorine Have 7 Electrons?
Fluorine has seven electrons because it has 9 total electrons, with two in the first energy level and seven in the second. This follows the octet rule, which states that atoms tend to gain, lose, or share electrons to reach a full valence shell.
What Is The Ionic Configuration Of F?
The ionic configuration of F is [He] 2s² 2p⁵.
Conclusion
Understanding fluorine’s electron configuration is essential for grasping its chemical properties. By knowing the arrangement of its 9 electrons, we can comprehend the element’s reactivity and bonding behavior. With this knowledge, we gain insights into how fluorine interacts with other elements, contributing to various applications in industry and everyday life.