Rubidium’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹, which indicates that it has 37 electrons distributed in different orbitals. Rubidium is a soft, silvery-white alkali metal that is highly reactive and can ignite spontaneously in air.
Rubidium is commonly used to manufacture vacuum tubes, photocells, and atomic clocks. Its properties make it valuable in scientific research, particularly in nuclear magnetic resonance spectroscopy. In addition, it has some potential to treat neurological disorders such as Parkinson’s disease.
With its unique chemical and physical properties, rubidium continues to be an important element in various fields of study.
The Basics Of Electron Configuration
Rubidium’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s¹, with the last electron occupying the fifth shell. The configuration depicts the arrangement of electrons in an atom’s energy level, promoting atomic properties’ understanding.
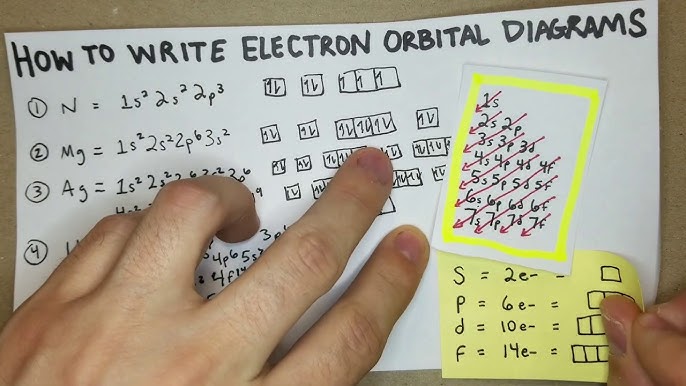
Electron configuration is the distribution of electrons in an atom or molecule in atomic or molecular orbitals. Understanding how electrons are arranged in an atom is essential in explaining how atoms behave chemically. Radium has gained attention with the atomic number 37 because of its unique electron configuration. This article aims to provide an overview of the basics of electron configuration and focuses on rubidium.
What Are Electrons?
Electrons are negatively charged particles that orbit the nucleus of an atom. They balance the positively charged protons in the nucleus, creating a stable atom. Electrons are always moving in an orbital or a shell around the nucleus. There can be one or more electrons in each orbital depending on the shell and the element. In general, the more electrons an element has, the more complex its electron configuration.
What Is a Valence Electron?
Valence electrons are the outermost electrons in an atom’s electronic configuration. They’re responsible for chemical bonding and reactivity. The valence electron configuration determines an atom’s chemical behavior, and a complete valence shell configuration corresponds to a stable and unreactive element. Each element has a unique number of valence electrons, which correspond to its position in the periodic table.
What Is The Electron Configuration Of An Atom?
The electron configuration of an atom describes how electrons are arranged in its atomic orbitals. Electrons fill the lowest energy orbitals first before moving to higher energy levels. A simplified representation of electron configuration is using noble gas shorthand or core notation. Rubidium’s electron configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s1. It has a total of 37 electrons, with one valence electron in the 5th shell.
In Conclusion
Understanding electron configuration is critical to understanding the chemical behavior of elements. Valence electrons determine an atom’s reactivity, while the electron configuration describes how electrons are distributed in an atom’s orbitals. Rubidium has a unique electron configuration, and by understanding its electron configuration, we can gain insight into its chemical properties.
Overview Of Rubidium
Rubidium is a soft, silvery-white metal with the symbol Rb and atomic number 37. It is grouped with lithium and sodium in Group 1A of the periodic table, also known as the alkali metals.
History Of Rubidium
Rubidium was first discovered in 1861 by German chemists Robert Bunsen and Gustav Kirchhoff during a spectroscopic analysis of mineral water. They detected two bright red lines corresponding to an unknown element in the sample. The element was eventually isolated and named rubidium, derived from the Latin word “rubidus,” meaning “deep red.”
Physical Properties Of Rubidium
Rubidium has a melting point of 39.3°C and a boiling point of 688°C. It is a highly reactive metal, tarnishing quickly in the air and reacting explosively with water. The element is soft and can be easily cut with a knife. Rubidium has a density of 1.53 grams per cubic centimeter and is the second most electropositive element after cesium.
|
Physical Properties of Rubidium |
|
|---|---|
|
Melting point |
39.3°C |
|
Boiling point |
688°C |
|
Density |
1.53 g/cm³ |
Chemical Properties Of Rubidium
Rubidium is highly reactive and easily forms compounds with other elements. It reacts vigorously with water, producing hydrogen gas and igniting the hydrogen. Rubidium also reacts with air, forming a layer of oxide on its surface. The element is a strong reducing agent, meaning it tends to give electrons to other elements. Rubidium has only one naturally occurring isotope, ^85Rb, but several other isotopes can be artificially produced.
-
Rubidium reacts with water to produce hydrogen gas
-
The element is highly reactive and forms compounds easily
-
Rubidium is a strong reducing agent
In conclusion, Rubidium is a highly reactive metallic element with a deep red color. Its discovery and subsequent isolation have contributed greatly to our understanding of spectroscopy and chemical reactions. As with many elements in the periodic table, rubidium has unique physical and chemical properties that make it useful in various applications, such as atomic clocks and battery technology.
Rubidium Electron Configuration
Rubidium’s electron configuration is [Kr] 5s1, meaning that its outermost electron is located in the s-orbital of the fifth energy level. This gives rubidium unique chemical properties that make it useful in various applications, including atomic clocks and biomedical research.
How is Rubidium Electron Configuration Written? Rubidium is a chemical element with an atomic number of 37, and its electron configuration is written in a specific way. The configuration defines how its electrons are distributed among the atomic orbitals and are represented by a series of numbers, letters, and superscripts. The electron configuration is written by starting with the lowest energy level and filling up each sublevel before moving to the next one. What is the Electron Configuration of Rubidium? The electron configuration of Rubidium is [Kr]5s1, where [Kr] represents the electron configuration of krypton and 5s1 represents the electron in the fifth energy level of the s sub-level. The electron configuration of Rubidium is unique and is different from other elements due to the addition of the 5s orbital. What is the Ground State Electron Configuration of Rubidium? Rubidium’s ground state electron configuration is the arrangement of its electrons in the lowest energy levels. The electron configuration of the ground state is represented by [Kr]5s1. Since Rubidium has only one electron in the outermost shell, it tends to lose that electron, which is why it is a highly reactive alkali metal. What is the Valence Electron Configuration of Rubidium? The valence electron configuration of Rubidium represents the outermost electrons available for chemical reactions. The valence electron configuration is also represented by [Kr]5s1 since Rubidium has only one valence electron in its outer shell. This configuration explains why Rubidium behaves similarly to other alkali metals, losing its valence electron and forming positive ions. In conclusion, the electron configuration of Rubidium is unique and defines how electrons are arranged. Understanding the electron configuration of an element is important in predicting its chemical behavior and chemical reactions. Knowing the electron configuration of Rubidium explains why it belongs to the alkali metal group and why it has a strong tendency to lose an electron.
Importance Of Rubidium Electron Configuration
Rubidium’s electron configuration is important as it determines the element’s chemical and physical properties. With a configuration of 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 5s¹, Rubidium has a single valence electron, which makes it highly reactive in chemical reactions.
Understanding its electron configuration is essential in predicting its chemical behavior.
Rubidium is one of the highly reactive alkali metals with atomic number 37 and symbol Rb. In a neutral rubidium atom, there are 37 protons and 37 electrons. The electron configuration of rubidium plays a crucial role in understanding this element’s physical and chemical properties. In this article, we will discuss the importance of rubidium electron configuration and how it affects the properties of this element.
How Does Rubidium Electron Configuration Affect Its Physical Properties?
Rubidium has a unique electron configuration with four energy levels, and the outermost shell has only one electron. The electron configuration of an element can affect its physical properties, such as density, melting point, boiling point, and atomic radius. Rubidium has a low melting point and boiling point due to weak metallic bonding between atoms, and its density is relatively low compared to other alkali metals. The low melting point of rubidium is due to its weak metallic bonding, and its boiling point is lower than the melting point due to a significant number of vapor molecules being generated by the free atoms. Rubidium’s atomic radius is relatively large, making it one of the most massive alkali metals. The large atomic radius of rubidium makes it more reactive, which means it reacts quickly with other elements to form compounds.
How Does Rubidium Electron Configuration Affect Its Chemical Properties?
The chemical properties of an element are primarily determined by its electron configuration. Rubidium’s electron configuration shows that it has only one electron in its outermost shell, making it highly reactive. Rubidium reacts with high vigor with air, water, and other elements, especially halogens, producing flammable hydrogen. Rubidium hydroxide (RbOH), a strong base, is formed when rubidium reacts with water. Rubidium has similar chemical properties to other alkali metals such as sodium, potassium, and cesium, but its properties are more pronounced due to its electron configuration. Rubidium is used in various applications, such as atomic clocks, photocells, and fireworks. In conclusion, the electron configuration of rubidium plays a significant role in determining its physical and chemical properties. The unique electron configuration of rubidium makes it highly reactive and affects its reactivity with other elements. Understanding the importance of rubidium electron configuration is essential in studying this element and its uses in different applications.
Applications Of Rubidium Electron Configuration
Rubidium’s electron configuration allows it to have unique properties that are useful in many applications. It’s often used in atomic clocks and as a source of positron emission in medical imaging. Its low ionization energy also makes it useful in spectroscopy and as a catalyst in organic synthesis reactions.
Rubidium is a soft, silvery-white metallic element in the alkali metal group. Its electron configuration plays an essential role in various applications across various industries. In this article, we will explore the significant areas where Rubidium Electron Configuration finds extensive use.
Rubidium Electron Configuration In Medical Applications
Rubidium Electron Configuration is widely used in nuclear medicine to diagnose cardiovascular diseases. Rubidium-82, a radioactive isotope of rubidium, is administered to patients as a positron emission tomography (PET) tracer. Its rapid decay and short half-life make it ideal for detecting blood flow changes in the heart, thereby aiding in diagnosing heart-related ailments.
Rubidium Electron Configuration In Industrial Applications
Rubidium is widely employed as a catalyst in the petrochemical industry, especially in the production of synthetic rubber and gasoline. Additionally, Rubidium Electron Configuration finds applications in manufacturing photocells, vacuum tubes, and atomic clocks. Its unique properties and high reactivity make it suitable for use in various types of sensors, including infrared sensors, pyrometers, and photoelectron emission sensors.
Rubidium Electron Configuration In Research
Rubidium Electron Configuration plays a critical role in various research applications, especially in the study of atomic and nuclear physics. Rubidium atoms are commonly used to study Bose-Einstein condensates, which are unique physical states of matter that occur at extremely low temperatures. Additionally, Rubidium Electron Configuration finds use in magneto-optical trapping techniques, which aids in studying laser cooling and trapping of atoms. In conclusion, Rubidium Electron Configuration is integral in various industries, including medical, industrial, and research. Its unique properties and reactivity make it suitable for a wide array of applications, from the production of synthetic rubber to the manufacture of atomic clocks.
Frequently Asked Questions Of Electron Configuration Rubidium
How Do You Write The Electron Configuration For Rubidium?
The electron configuration for Rubidium is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹.
What Is The Electron Configuration Of 1s2 2s2 2p6 3s2 3p6 4s2?
The electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 is how electrons are arranged in an atom. It has 2 electrons in the 1s orbital, 2 in the 2s orbital, 6 in the 2p orbital, 2 in the 3s orbital, 6 in the 3p orbital, and 2 in the 4s orbital.
What Element Has An Electron Configuration Of 1s 2 2s 2 2p 6 3s 2 3p 3?
The element with an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 3 is phosphorus (P), which has the atomic number 15.
What Is The Element Configuration For 1s 2 2s 2 2p 6 3s 2 3p 4?
The element has 16 valence electrons and belongs to the third period and fourth group of the periodic table. Its atomic number is 16, and the element is sulfur. The element configuration is 1s2 2s2 2p6 3s2 3p4.
Conclusion
Understanding rubidium’s electron configuration is essential to comprehending its chemical properties and behavior. By analyzing its placement on the periodic table and its outermost electron shell, we can discern its reactivity and the type of bonds it forms.
This knowledge can be applied in various fields, including nuclear medicine, lighting technology, and even studying the Earth’s crust. As we continue to explore the mysteries of the universe, studying electron configurations will be an important tool in unraveling its secrets.