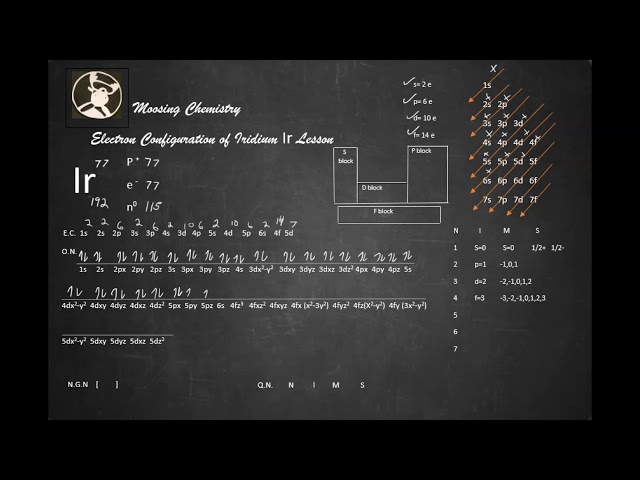
Iridium’s electron configuration is [Kr] 4d^7 5s^2. Iridium is a transition metal with the atomic number 77 and is a member of the platinum group of metals.
Iridium is a very dense, hard, and brittle metal with a white-gold color. It is extremely resistant to heat, wear, and corrosion and has a high melting point, making it useful in alloys, electrical contacts, and catalysts. Due to its rarity and durability, it is also used to produce fountain pen nibs and high-end jewelry.
Iridium is an important element in various fields because of its wide range of applications in industry and technology.
What Is Iridium?
Iridium is a chemical element with an atomic number of 77 and the symbol Ir. Its electron configuration is [Xe] 4f14 5d7 6s2, with a total of 77 electrons. This transition metal is known for its dense, hard, and corrosion-resistant properties, and it is often used in electronics and catalytic converters.
Iridium is a chemical element with the symbol Ir. It belongs to the group of transition metals and has an atomic number of 77. The name “Iridium” comes from the Latin word “iris,” which means rainbow, because of its colorful compounds. Iridium is a hard, dense, silvery-white metal that is the second-densest element after osmium. Iridium is also classified as a noble metal due to its excellent resistance to corrosion and oxidation. In this article, we will explore the electron configuration of Iridium and its significance. In simple terms, electron configuration refers to the distribution of electrons of an atom in its atomic orbitals. The orbitals of an atom are the regions around the nucleus where electrons are found. The electron configuration of an atom is represented in a specific notation that includes numbers, letters, and superscripts.
Electron Configuration Of Iridium
The electron configuration of Iridium can be written in shorthand notation using the noble gas configuration method. The noble gas configuration method involves representing the electron configuration of an element using a noble gas closest to it in the periodic table. For example, the electron configuration of Iridium can be written as [Xe] 4f14 5d7 6s2. Here’s another way to represent it:
|
Subshell |
Electrons |
|---|---|
|
1s |
2 |
|
2s |
2 |
|
2p |
6 |
|
3s |
2 |
|
3p |
6 |
|
4s |
2 |
|
3d |
10 |
|
4p |
6 |
|
5s |
2 |
|
4d |
7 |
|
5p |
1 |
From the electron configuration of Iridium, we can see that it has nine electrons in its 5D subshell and seven electrons in its 4D subshell, making it an element of interest to researchers studying transition metal chemistry. In conclusion, Iridium is a chemical element with unique physical and chemical properties. Understanding its electron configuration provides insight into its reactivity and potential applications.
What Is Electron Configuration?
Electron configuration refers to how electrons are arranged in an atom’s energy levels. Iridium, a transition metal, has an electron configuration of [Kr] 4d^10 5s^2 5p^6 5d^7, where Kr represents the noble gas core electron configuration.
A Concise Explanation Of Electron Configuration
Electron configuration refers to the arrangement of electrons in an atom’s orbitals. In simpler terms, it describes how electrons are distributed in an atom’s energy levels. Understanding electron configuration is crucial in predicting the chemical behavior of elements.
The Significance Of Electron Configuration
The arrangement of electrons in an atom plays a significant role in how an atom bonds with other atoms. By simply examining the electron configuration of an atom, one can predict its reactivity and group it accordingly in the periodic table.
How To Write Electron Configuration
Electron configuration is written by denoting the principal quantum number, which represents the energy level, followed by the orbital letter that describes the sublevel, and the superscript that denotes the number of electrons in that sublevel. For example, the electron configuration of iridium, a transition metal, is [Xe] 4f14 5d7 6s2. This configuration denotes that it has five energy levels, with electrons distributed across the 4f, 5d, and 6s orbitals.
Exceptions To The Standard Electron Configuration
There are exceptions to the standard electron configuration rules, such as the half-filled and filled subshells, which have lower energy levels due to the stable configuration. For instance, the chromium atom has a configuration of [Ar] 3d5 4s1 instead of [Ar] 3d4 4s2, which is a more stable configuration. In summary, electron configuration describes how electrons are distributed in an atom’s energy levels and play an important role in predicting the chemical behavior of elements. Writing electron configuration involves denoting the principal quantum number, orbital letter, and the number of electrons. Exceptions to the standard configuration exist, such as filled and half-filled subshells.
Why Is Electron Configuration Important For Iridium?
Iridium has a complex electron configuration with 9 valence electrons. This configuration is important because it affects the chemical and physical properties of Iridium, making it valuable in industrial applications such as catalysts and electronics.
The Relevance Of Electron Configuration For Iridium’s Properties
As an element, Iridium has numerous applications, including in the production of electrical components and alloys for turbine engines. However, its unique properties can be attributed to its electron configuration. Iridium has an atomic number of 77, and its electron configuration is [Kr] 4d^7 5s^2. This means that Iridium has nine valence electrons, and its outermost electron shell is incomplete, which makes it more susceptible to chemical bonding.
Electron Configuration’s Impact On Iridium’s Chemical Behaviour
Electron configuration has a direct impact on an element’s chemical behavior. As mentioned above, Iridium has nine valence electrons, and a completed outermost shell will require one more electron. Alternatively, Iridium’s incomplete outermost shell makes it easier to bond with other elements to complete the shell. This unique chemical property makes Iridium highly reactive when bonding with other elements, particularly those that require nine valence electrons to complete their outermost shell. The high reactivity of Iridium is one of its most unique properties, and it is highly valuable in various industries.
Electron Configuration And Iridium’s Electronic Properties
The electron configuration of Iridium also gives it unique electronic properties. For instance, its electron configuration is responsible for its high melting and boiling point, making it an ideal material for high-temperature applications and electrical conductivity. Furthermore, Iridium has a high density, making it valuable for a wide range of uses, such as in electronics, where high-density materials are a necessity. The electronic properties of Iridium make it an indispensable element in several industries, highlighting the importance of its electron configuration. In summary, the unique properties of Iridium and its electron configuration explain why it is an essential element for many industries. Its incomplete outermost shell makes it highly reactive, while its electronic properties are responsible for its high density and high-temperature stability. Therefore, the electron configuration of Iridium is crucial for determining its chemical and physical properties and its suitability for various applications.
What Is The Electron Configuration Of Iridium?
Iridium is a transition metal with an electron configuration of [Xe] 4f14 5d7 6s2. This configuration indicates that out of the nine electrons present in the 5d orbital, seven are filled, and two are unpaired, forming a half-filled subshell. This arrangement contributes to its various unique properties, including its high melting and boiling points and resistance to corrosion.
Iridium is a chemical element with the symbol Ir and an atomic number of 77. It’s a transition metal known for its high melting point, resistance to corrosion, and use in various applications, including fountain pen tips, crucibles, and spark plugs. Iridium’s electron configuration is an essential aspect of its chemical reactivity and chemical bonding. Knowing the electron configuration of iridium is essential in predicting how it will react with other elements and form different chemical compounds.
The Complete Electron Configuration Of Iridium
The complete electron configuration of iridium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d7 6s2. This notation represents the distribution of electrons in the different energy levels or orbitals of the iridium atom. Each orbital can hold a specific number of electrons, with the first energy level capable of holding two electrons, the second level eight electrons, the third level 18 electrons, the fourth level 32 electrons, and the fifth energy level can hold up to 50 electrons.
Breaking Down Iridium’s Electron Configuration
Iridium’s electron configuration can be broken down into its different energy levels or orbitals. The first energy level, or the K shell, has two electrons, with the electrons occupying the s orbital. The second energy level, or L shell, has eight electrons, and the s and p orbitals are filled. The third energy level, or M shell, has 18 electrons, with the s, p, and d orbitals complete. The fourth energy level, or N shell, has 32 electrons, and all the orbitals are filled except for the s and d orbitals, which have two and one electron, respectively. The fifth energy level, or O shell, has nine electrons in the s and p orbitals and seven electrons in the d orbital, with the remaining electrons occupying the f orbital. In iridium, the 5d orbital is the highest energy orbital with electrons. The irregularity in the electron configuration of iridium is due to the presence of the f orbitals with higher energy than the d orbitals. In summary, iridium’s electron configuration is an essential aspect of the element’s chemical behavior. It can be represented as 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d7 6s2. Understanding the different energy levels and orbitals can help predict the element’s chemical reactions and the formation of different compounds.
How Does The Electron Configuration Affect Iridium’s Properties?
The electron configuration of Iridium significantly impacts its properties, both physical and chemical. Iridium is a transition metal with an atomic number of 77. Its electron configuration is [Xe] 4f14 5d7 6s2. This electronic configuration plays a crucial role in how Iridium behaves chemically.
The Influence Of Electron Configuration On Iridium’s Physical And Chemical Properties
Many physical and chemical properties of Iridium can be explained by its electron configuration. For example, the presence of seven electrons in the 5d orbital makes Iridium particularly hard and dense. In fact, it is one of the densest natural elements. Its high melting point, boiling point, and corrosion resistance are also due to its electronic configuration.
Reactivity And Bonding Based On Iridium’s Electron Configuration
Iridium’s electron configuration also influences its reactivity and bonding. The 5d electrons are located far from the nucleus, which makes it more prone to oxidation. Despite being highly corrosion-resistant, Iridium can still form compounds with oxygen and halogens. Its seven outer electrons make it a versatile element with diverse bonding properties.
The configuration of Iridium’s electrons plays a vital role in making it an ideal catalyst in various chemical reactions. The 5d orbitals have a unique shape that allows them to form chemical bonds with other atoms. This unique electronic configuration is responsible for Iridium’s exceptional catalytic activity in various chemical reactions, including the hydrogenation of organic compounds.
In conclusion, the electron configuration of Iridium plays a significant role in determining its physical and chemical properties. It contributes to the element’s density, hardness, resistance to corrosion, reactivity, and bonding properties. With its unique electronic configuration, Iridium is an essential element in many industrial applications, particularly in catalysis.
Applications Of Iridium’s Electron Configuration
Iridium is a chemical element with the symbol Ir and atomic number 77. It is a dense, silvery-white transition metal with various applications across different fields. Iridium’s electron configuration determines its distinct chemical and physical properties, which make it an essential element in modern industries.
Applications Of Iridium In Electronics
Iridium has unique properties that make it ideal for use in various electronic devices, including laptop hard disks, mobile phones, and LEDs. Due to its high melting point, Iridium can withstand high temperatures and is suitable for use in high-temperature applications, such as spark plugs in vehicles. Additionally, its small size makes it useful in microelectronics, where tiny components are required.
Applications Of Iridium In Catalysis
Iridium is also an important catalyst in various chemical reactions, including hydrogenation, oxidation, and isomerization. It is used in the chemical industry to speed up reactions and convert one substance into another. Iridium catalysts are widely used to produce polymers, drugs, and other chemicals.
Applications Of Iridium In Biomedicine
The excellent biocompatibility of Iridium makes it ideal for use in biomedical applications. Studies have shown that Iridium complexes can have potential anticancer properties, where they target and destroy cancer cells. Iridium compounds have also been found to be effective against other diseases, such as malaria, Alzheimer’s, and Parkinson’s.
In conclusion, Iridium’s unique electron configuration makes it an essential element with diverse applications in various fields, such as electronics, catalysis, and biomedicine. Its distinct properties make it a valuable material in modern industries, and researchers continue to explore its potential applications in various fields.
Iridium And The Periodic Table
The periodic table is a tabular arrangement of chemical elements, ordered by their atomic number, electron configurations, and chemical properties. It provides essential information about every known element, such as its symbol, name, atomic mass, and number, among others. Iridium, with an atomic number of 77, is one of the elements in the periodic table. This rare and lustrous metal is a member of the platinum group of elements and has several unique physical and chemical properties, including its electron configuration.
Iridium’s Position In The Periodic Table
Iridium belongs to the third-row transition metals of the periodic table, situated between platinum and osmium. It is also part of the d-block section or the middle block of elements corresponding to the filling of d orbitals in their electron configurations. Its position in the periodic table shows some of its properties, including its chemical reactivity, melting point, and atomic size.
Relation Between Iridium’s Electron Configuration And Its Position In The Periodic Table
An element’s electron configuration provides valuable information about its chemical properties, including its valence electrons, oxidation state, and reactivity. Iridium’s electron configuration is [Xe] 4f14 5d7 6s2, indicating that it has nine valence electrons and is part of the eighth group of elements. Its electron configuration also shows its stability and high density, which makes it indispensable in various industrial applications.
In conclusion, understanding Iridium’s electron configuration and its position in the periodic table provides a wealth of information on its chemical and physical properties. Knowing these properties is vital in developing new applications of Iridium, especially in fields such as electronics, aerospace, and medicine, where its unique characteristics make it an essential element.
Frequently Asked Questions Of Electron Configuration Iridium
What Is The Atomic Notation For Iridium?
Iridium’s atomic notation is Ir, its atomic number is 77, and its atomic weight is 192. 217.
How Many Electrons Are There In Iridium?
Iridium contains 77 electrons.
What Is Rn 7s2 5f14 6d4?
RN 7s2 5f14 6d4 refers to the electron configuration of an atom, specifically of an element with 101 protons. It shows how the electrons are arranged in the atom’s orbitals.
What Is The Electron Configuration Of Radium?
The electron configuration of radium is [Rn] 7s².
Conclusion
Iridium’s anomalous electron configuration contributes to its unique chemical properties and extensive use in various industrial applications. Its impressive physical properties make it a critical element for various electronic devices, including solar cells and durable hard disks. As we continue to explore the intricate nature of iridium, it’s clear that this transition metal will play a pivotal role in advancing future technologies and innovations.
By understanding iridium’s electron configuration, we can harness its potential and push the scientific boundaries further.