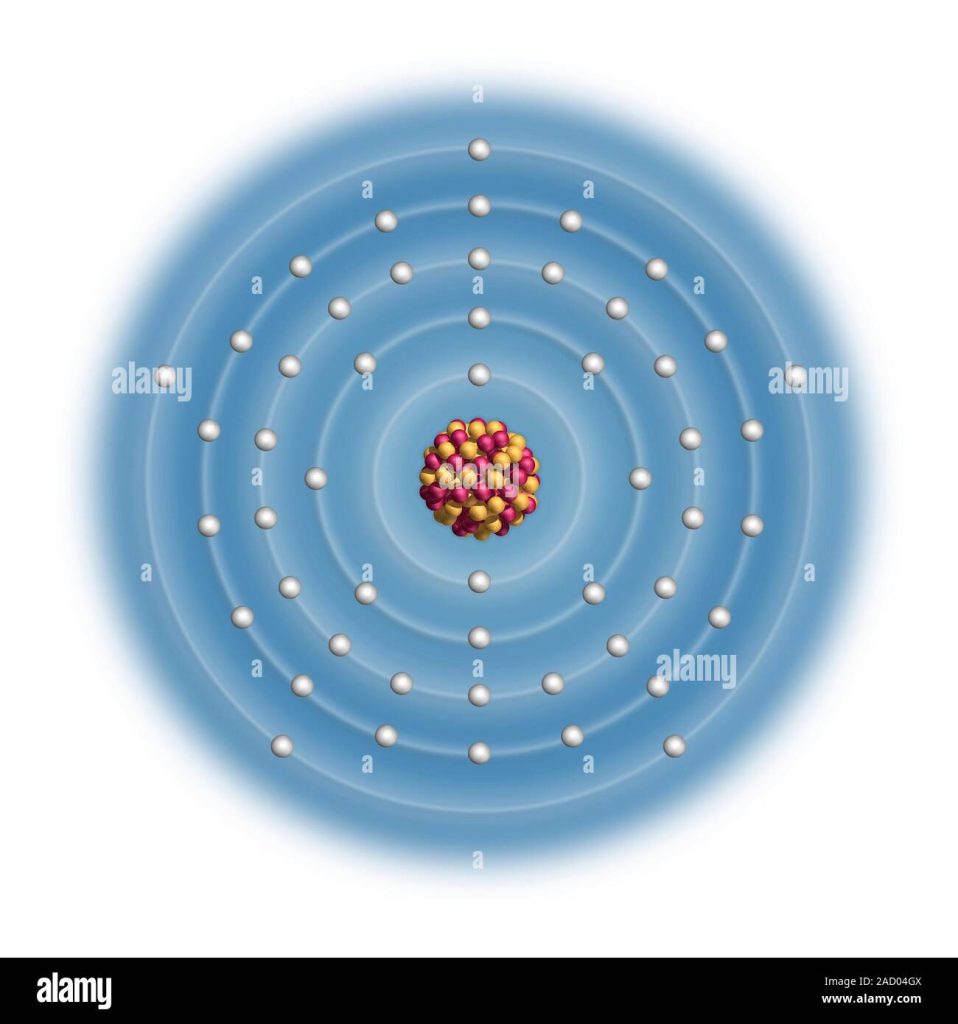
The Antimony Electron Configuration is [Kr] 4d^10 5s^2 5p^3. Antimony, a chemical element with atomic number 51, belongs to group 15 of the periodic table and is commonly found in nature as a brittle, silvery-white metal.
Its electron configuration consists of the noble gas kernel of krypton, followed by the filling of the 4d and 5s orbitals and ending with three electrons in the 5p orbital. This electron arrangement determines the chemical properties and behavior of antimony, making it an important element in various applications such as metallurgy, medicine, and electronics.
We will explore the electron configuration of antimony in more detail and discuss its significance in understanding the element’s characteristics.
Understanding Electrons
Antimony’s electron configuration plays a fundamental role in understanding electron behavior. By comprehending the arrangement of electrons in the antimony atom, scientists can explore its chemical properties, bonding, and reactivity in various environments. This knowledge is a crucial foundation for exploring its applications in different fields.
Understanding Electrons The atomic structure consists of protons, neutrons, and electrons. In this blog post, we will delve into the electron configuration of antimony and gain a better grasp of this fundamental aspect of chemistry. The Structure of an Atom The atom’s structure comprises a nucleus at the core, encompassing protons and neutrons. Electrons revolve around the nucleus in specific energy levels or shells, each with a maximum number of electrons it can accommodate. These shells are labeled K, L, M, N, and so on, with the closest shell to the nucleus being K. As we move away from the nucleus, the energy levels increase, allowing the placement of more electrons. The Role of Electrons Electrons is critical in determining the chemical behavior of an element. They are involved in forming chemical bonds, and the arrangement of electrons in an atom influences its properties. The electron configuration of an element provides a roadmap to understanding its reactivity, stability, and bonding behavior with other elements. In the case of antimony, comprehending its electron configuration is essential in predicting its chemical characteristics. In conclusion, understanding the role and arrangement of electrons within an atom is crucial for comprehending the chemical behavior of elements. The atom’s structure, particularly the placement of electrons, plays a significant role in determining an element’s properties and reactivity. This knowledge is essential in various scientific fields, from chemistry to material science and beyond.
The Periodic Table
Antimony’s electron configuration can be found on the periodic table, providing essential information about its electron arrangement. By understanding its electron configuration, scientists can gain valuable insights into antimony’s chemical properties and behavior.
The periodic table is one of the most essential tools in the field of chemistry. It is a systematic arrangement of all the known elements in the universe, providing valuable information about their properties and behaviors. This organized chart has simplified scientists’ understanding of elements, facilitating their studies and discoveries.
Organization Of Elements
The elements in the periodic table are arranged based on their atomic numbers, electron configurations, and chemical properties. The table consists of rows, called periods, and columns, called groups or families. This systematic organization allows scientists to easily identify patterns and trends among elements, aiding their research and experimentation.
Groups And Periods
The periodic table is divided into groups and periods, each with unique characteristics and properties. Groups are the vertical columns numbered from 1 to 18. These groups share similar chemical properties, with the same number of valence electrons. Valence electrons are crucial in determining an element’s reactivity and bonding behavior.
Periods, on the other hand, are the horizontal rows in the periodic table. There are seven periods in total, each corresponding to a new energy level or shell in an atom. As you move from left to right across a period, the atomic number increases, indicating the addition of more protons and electrons.
Understanding the arrangement of elements in groups and periods helps scientists anticipate their behavior and predict their interactions with other elements. This knowledge becomes especially crucial when studying electron configurations, as the position of an element in the periodic table provides valuable insights into the arrangement of its electrons within its atomic structure.
The Formation Of Ions
When it comes to exploring the electron configuration of antimony and its role in forming ions, it’s important to understand the concept of ions first. Ions are atoms or molecules that have gained or lost electrons, resulting in a positive or negative charge. In the case of antimony, its electron configuration determines the number of electrons available for the formation of ions.
Positively Charged Ions
Positively charged ions, or cations, are formed when an atom loses one or more electrons. In the electron configuration of antimony, its outermost shell contains five electrons. However, to achieve a stable configuration, antimony tends to lose these electrons and form positively charged ions.
The formation of positively charged antimony ions involves the loss of electrons from the outermost shell. For example, antimony can lose three electrons, resulting in a +3 charge. This transformation occurs when antimony comes into contact with certain elements or compounds with a higher affinity for electrons.
The electron loss allows the antimony atom to achieve a stable electron configuration similar to that of noble gases. This stability is important for the ion’s overall balance and reactivity.
Negatively Charged Ions
Negatively charged ions, also known as anions, are formed when an atom gains one or more electrons. Antimony has the potential to gain electrons due to its electron configuration, which leaves it with three empty spaces in the outermost shell.
The gain of electrons by antimony enables it to achieve a stable electron configuration by filling the empty spaces in its outermost shell. For example, antimony can gain three electrons to attain a -3 charge. This process occurs when antimony interacts with elements or compounds that have a tendency to donate or transfer electrons.
The presence of extra electrons in the outermost shell affects the ion’s chemical and physical properties. It can influence the ion’s reactivity, solubility, and ability to form chemical bonds with other atoms.
In conclusion, antimony’s electron configuration is pivotal in forming positively and negatively charged ions. Understanding these processes allows us to comprehend the behavior and characteristics of antimony ions in various chemical reactions and applications. Google maps
Frequently Asked Questions For Antimony Electron Configuration
How Do You Write The Electron Configuration For Antimony?
The electron configuration for antimony is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^2 5p^3.
Which Element Has The Electron Configuration Of 1s 2 2s 2 2p 6 3s 2 3p?
The element with the electron configuration 1s2 2s2 2p6 3s2 3p is silicon.
What Element Has The Electron Configuration 1s22s22p63s23p3?
The element with the electron configuration 1s22s22p63s23p3 is phosphorus (P).
What Is The Electron Configuration Of The Element 51?
The electron configuration of element 51 is [Kr] 5s^24d^105p^3.
Conclusion
Understanding the electron configuration of antimony is crucial for understanding its properties and behaviors. By delving into the arrangement of its electrons, we gain valuable insight into the element’s chemical and physical characteristics. With this knowledge, we can further explore its applications and significance, contributing to advancements in various scientific fields.