Neon has eight valence electrons. Neon is a noble gas in Group 18 of the periodic table.
Neon, symbolized as Ne, is a colourless, odourless noble gas that belongs to Group 18 of the periodic table. It is known for its high chemical inertness and stability due to its complete outer electron shell. Neon has ten electrons, eight of which are in its outermost shell, making it highly stable and non-reactive.
This characteristic makes neon useful in various applications, including neon signs, high-voltage indicators, and vacuum tubes. Its bright, vibrant glow when electrically charged is a hallmark of neon lights, commonly seen in advertising and artistic displays. Due to its unique properties, neon plays a crucial role in many industries.
Introduction To Neon
Neon is a fascinating element in the periodic table. It is renowned for the vivid, bright lights used in its signs. This post explores the valence electrons of Neon. Understanding these electrons helps explain Neon’s unique properties.
Elemental Properties
Neon is a noble gas with the symbol Ne and atomic number 10. In its natural state, it has no colour, smell, or taste. Neon is the fifth most abundant element in the universe. Here are some key properties of Neon:
- Atomic Number: 10
- Atomic Weight: 20.18
- Boiling Point: -246.08°C
- Melting Point: -248.59°C
- Density: 0.9002 g/L
Significance In The Periodic Table
Neon belongs to the noble gases group, which is group 18. Noble gases are known for their stability and lack of reactivity. This stability is due to their complete valence electron shells. neon has eight valence electrons, making its outer shell full. This full shell gives neon its lack of reactivity. Here are some other noble gases:
- Helium (He)
- Argon (Ar)
- Krypton (Kr)
- Xenon (Xe)
- Radon (Rn)
Neon’s place in group 18 highlights its stability and unique properties, which make it a critical element in scientific studies and applications.
Atomic Structure
The atomic structure of an element reveals its unique properties. Understanding this structure helps in knowing how elements behave and react.
Protons, Neutrons, And Electrons
Atoms consist of three main particles: protons, neutrons, and electrons.
- Protons: Positively charged particles in the nucleus.
- Neutrons: Neutral particles are also in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus.
Neons have 10 protons and usually 10 neutrons.
Electron Shells And Orbitals
Electrons orbit the nucleus in defined paths known as shells or orbitals. Each shell can hold a specific number of electrons.
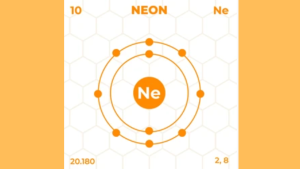
Neon’s electrons are arranged in two shells:
| Shell | Maximum Electrons |
|---|---|
| First Shell | 2 |
| Second Shell | 8 |
Neon’s electron configuration is 2 in the first shell and 8 in the second shell. This means Neon has 8 valence electrons.
Valence Electrons Explained
Understanding valence electrons is key to learning chemistry. Valence electrons play a crucial role in chemical reactions and bonding. Let’s explore what valence electrons are and their significance.

Definition Of Valence Electrons
Valence electrons are the electrons in the outermost shell of an atom. They determine how an atom interacts with other atoms. These electrons are involved in forming bonds.
Role In Chemical Reactions
Valence electrons dictate how elements react. Atoms with a full valence shell are stable. Neon has 8 valence electrons, making it very stable.
| Element | Valence Electrons | Reactivity |
|---|---|---|
| Neon | 8 | Non-reactive |
| Oxygen | 6 | Highly reactive |
| Sodium | 1 | Very reactive |
Neon’s 8 valence electrons make it inert, so it does not easily form compounds. This stability is why neon is used in signs and lights.
- Valence electrons are key for understanding chemical properties.
- Atoms seek to have a full valence shell.
- Neon, with 8 valence electrons, is very stable.
Neon’s Electron Configuration
Neon is a noble gas with a simple yet interesting electron configuration. Understanding this helps in understanding its chemical properties. Neon is known for its stability and lack of reactivity.
Electron Distribution
The electron configuration of neon can be written as 1s2 2s2 2p6. This notation shows the distribution of electrons in different orbitals. Neon’s atomic number is ten, and it has ten electrons distributed in its orbitals. The distribution follows the rule of filling up lower energy levels first.
| Orbital | Number of Electrons |
|---|---|
| 1s | 2 |
| 2s | 2 |
| 2p | 6 |
Neon’s electrons fill up the 1s orbital first, followed by the 2s and then the 2p orbitals. This complete filling makes neon very stable.
Energy Levels
The electrons in neon are arranged in two main energy levels. The first energy level, or shell, contains 2 electrons, and the second energy level contains 8 electrons. Each level is filled in a specific order.
- First energy level: 1s2
- Second energy level: 2s2 2p6
The fully filled second energy level gives neon its chemical inertness. Neon’s valence electrons are in the outermost shell. In neon, this shell has 8 electrons, which makes it stable.
Neon’s Valence Electrons
Neon is a noble gas with unique properties. Understanding neon’s valence electrons helps explain these properties. This section explores the valence electron count and its implications for reactivity.
Valence Electron Count
Neon has 10 electrons in total. These electrons are arranged in two shells. The first shell holds 2 electrons. The second shell holds 8 electrons. This means neon has 8 valence electrons. A full outer shell makes neon very stable.
Implications For Reactivity
Because neon has a full outer shell, it is very unreactive. Neon doesn’t easily form compounds with other elements. This stability makes neon perfect for neon signs and lighting. Its lack of reactivity is a key feature of all noble gases.
Comparing Noble Gases
Noble gases are unique. They are known for their stability. This is because of their valence electrons. Let’s compare Neon, Helium, and Argon.
Neon Vs. Helium
neon has ten electrons in total. It has eight valence electrons. This fills its outer shell. Neon is very stable.
Helium has two electrons. It has two valence electrons. Though fewer, its outer shell is also full. This makes Helium stable, too.
| Noble Gas | Total Electrons | Valence Electrons |
|---|---|---|
| Neon | 10 | 8 |
| Helium | 2 | 2 |
Neon Vs. Argon
Argon has eighteen electrons, and like neon, it has eight valence electrons. Both have full outer shells, which makes them stable.
The main difference is in the total number of electrons. Argon has more electrons than Neon.
| Noble Gas | Total Electrons | Valence Electrons |
|---|---|---|
| Neon | 10 | 8 |
| Argon | 18 | 8 |
Neon, Helium, and Argon are all stable. This is due to their full outer electron shells.
Real-world Applications
Neon, known for its atomic number 10 and eight valence electrons, has many practical uses. Its unique properties make it valuable in various industries.
Neon Lights
Neon lights are perhaps the most famous use of neon. These lights are used in signs, displays, and decorations.
- Bright Colors: Neon gas emits a bright, red-orange glow.
- Long-lasting: Neon lights last for years, making them cost-effective.
- Energy Efficient: They use less power than traditional lights.
Neon lights have become iconic, especially in urban landscapes and advertising. Their distinct glow is hard to miss.
Other Uses
Neon has many other applications beyond lighting.
- Cryogenics: Neon is used as a refrigerant in cryogenic applications.
- Lasers: Neon gas is essential in helium-neon lasers.
- High-voltage Indicators: Neon is used in high-voltage indicators and vacuum tubes.
Neon is also used in neon signage and television tubes.
Its stable, inert nature makes it safe for these uses. Google maps
Frequently Asked Questions
How Many Valence Electrons In Neon?
Neon has 8 valence electrons, which complete its outer shell. This makes It very stable, and it rarely forms compounds.
Why Does Neon Have 8 Valence Electrons?
Neon has 8 valence electrons because it is in Group 18. This group is also known as the noble gases. They all have full outer shells.
Is Neon Reactive With Other Elements?
Neon is not reactive. It has a full outer electron shell. This makes it stable and inert. It rarely forms compounds.
What Is The Electron Configuration Of Neon?
The electron configuration of Neon is 1s² 2s² 2p⁶. This shows that it has 2 electrons in the first shell and 8 in the second.
Conclusion
Understanding neon’s valence electrons helps grasp its chemical properties. Neon has 8 valence electrons, making it stable. This stability is why neon rarely forms compounds. Knowing these facts enriches your grasp of chemistry. Keep exploring the fascinating world of elements to deepen your knowledge.
Stay curious and keep learning!
