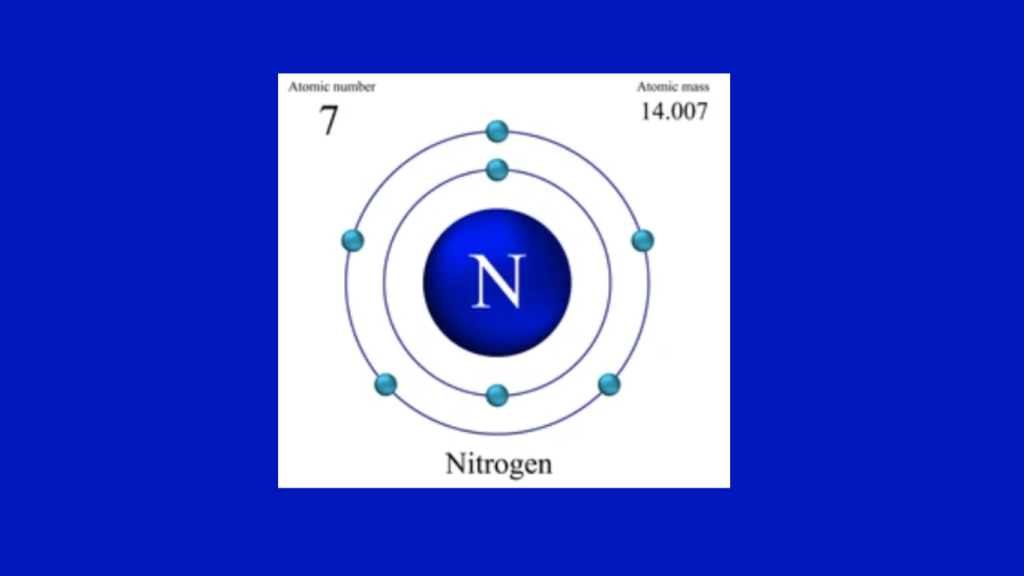
The Nitrogen Electron Configuration is 1s2 2s2 2p3. Nitrogen has 7 electrons.
Nitrogen, with an atomic number of 7, is a non-metallic element in the periodic table. It is known for its diatomic nature, forming stable N2 molecules. Nitrogen’s electron configuration is 1s2 2s2 2p3, indicating that it has two electrons in the 1s orbital, two in the 2s orbital, and three in the 2p orbital.
This arrangement gives nitrogen a half-filled p orbital, making it relatively stable. Nitrogen is essential for life as it is a crucial component of amino acids, proteins, and DNA. It plays a vital role in various biological and industrial processes, including nitrogen fixation and the production of fertilizers. Additionally, nitrogen gas is inert and commonly used in industrial applications to prevent oxidation and provide an inert atmosphere.
Electron Configuration Basics
Understanding an atom’s electron configuration is crucial to comprehending its chemical properties and behavior. The arrangement of electrons in an atom’s energy levels or shells holds significant importance in science. Let’s delve into the basics of electron configuration and its determination.
What Is Electron Configuration?
Electron configuration refers to the distribution of electrons in the atomic orbitals of an atom. It is a shorthand method for representing the arrangement of electrons in an atom. The electron configuration of an atom is described as a sum of the electrons in each energy level and sublevel.
How Is Electron Configuration Determined?
- The electron configuration of an atom is determined based on the Aufbau principle, which states that electrons occupy the lowest energy orbitals available.
- The Pauli exclusion principle and Hund’s rule further govern the distribution of electrons in different orbitals and sublevels.
- The pdf notation can represent the electron configuration, which signifies the different sublevels in an atom.
The Electron Configuration Of Nitrogen
Nitrogen is a chemical element with the symbol N and atomic number 7. Understanding its electron configuration is crucial to comprehending its chemical properties and behavior. An atom’s electron configuration describes the arrangement of its electrons within different energy levels and orbitals.
The Atomic Number Of Nitrogen
The atomic number of nitrogen is 7. This signifies that nitrogen possesses 7 protons and, consequently, 7 electrons in its neutral state. Protons carry positive charges, while electrons carry negative charges. The atomic number determines an element’s position within the periodic table, distinguishing it from other elements.
The Orbital Diagram Of Nitrogen
The orbital diagram of nitrogen showcases the distribution of its electrons within energy levels and orbitals. Nitrogen has two energy levels: the first energy level can accommodate a maximum of 2 electrons, while the second energy level can hold up to 8 electrons.
| Energy Level | Orbital | Electron Configuration |
|---|---|---|
| 1 | s | 1s2 |
| 2 | s | 2s2 |
| 2 | p | 2p3 |
The Electron Configuration Notation Of Nitrogen
The electron configuration notation of nitrogen is 1s2 2s2 2p3. This notation represents the specific occupation of each orbital by nitrogen’s electrons. The superscript numbers indicate the number of electrons present in each orbital, while the letters signify different atomic orbitals.
In the case of nitrogen, the notation reveals that the 1s orbital contains 2 electrons, the 2s orbital contains 2 electrons, and the 2p orbital contains 3 electrons. The electron configuration notation provides valuable insight into the arrangement of electrons in an atom, aiding chemists in understanding and predicting chemical reactions.
Understanding Energy Levels And Orbitals
Understanding the nitrogen electron configuration helps us comprehend energy levels and orbitals, contributing to a deeper knowledge of its properties. This understanding is crucial in studying the behavior and reactions of nitrogen in various chemical processes.
Energy Levels In An Atom
Energy levels play a crucial role in understanding the electron configuration of nitrogen. In an atom, electrons occupy specific energy levels, also known as shells or principal quantum numbers. Electrons occupy the lowest energy level available before moving to higher levels. The electron’s distance from the nucleus increases as the energy level increases.
Orbitals And Their Shapes
Orbitals provide a more nuanced understanding of how electrons are distributed within an atom’s energy levels. In simple terms, an orbital is a three-dimensional region where an electron is most likely to be found. Each energy level contains different types of orbitals named s, p, d, and f, each with its own unique shape. The s orbital has a spherical shape, while the p orbital has a dumbbell-like shape with three orientations: px, py, and pz. The d and f orbitals are more complex, with multiple shapes and orientations.
Filling Orbitals With Electrons
Filling orbitals with electrons follows specific rules. The Aufbau principle states that electrons first fill the lowest energy level before moving to higher energy levels. The Pauli exclusion principle states that each orbital can hold a maximum of two electrons with opposite spins. Hund’s rule states that electrons will occupy orbitals with the same energy level singly before pairing up. These principles help determine the electron configuration of nitrogen, which has seven electrons.
The Aufbau Principle
The Aufbau Principle is a fundamental concept in chemistry that explains how electrons are arranged in an atom. This principle is crucial for understanding the electron configuration of elements, including the nitrogen atom. By grasping the intricacies of the Aufbau Principle, one can gain valuable insights into the behavior of nitrogen and its chemical properties.
What Is The Aufbau Principle?
The Aufbau Principle, derived from the German word ‘Aufbau’ meaning “building up,” states that electrons fill atomic orbitals in order to increase energy levels. Simply put, electrons occupy the lowest energy levels first before filling higher ones, aligning with the concept of energy minimization in atomic structures.
Application Of The Aufbau Principle To Nitrogen
When applying the Aufbau Principle to nitrogen, which has an atomic number of 7, we consider the arrangement of its electrons within the electron configuration. Nitrogen’s electron configuration can be represented as 1s2 2s2 2p3, following the sequence dictated by the Aufbau Principle. This configuration reflects the filling of the 1s orbital before moving to the 2s and 2p orbitals, adhering to the principle of electron ordering based on energy levels.
The Pauli Exclusion Principle
The Pauli Exclusion Principle governs the electron configuration of nitrogen, preventing two electrons from occupying the same quantum state. This principle plays a crucial role in understanding the chemical properties and behavior of nitrogen.
What Is The Pauli Exclusion Principle?
The Pauli Exclusion Principle is a fundamental principle in quantum mechanics named after the renowned physicist Wolfgang Pauli. According to this principle, no two electrons in an atom can have the same quantum state. This means that if two electrons are occupying the same orbital, they must have different quantum numbers, which describe properties such as spin and energy level.
Implications For The Electron Configuration Of Nitrogen
The electron configuration of an atom describes how its electrons are distributed among different energy levels and orbitals. Understanding the Pauli Exclusion Principle helps us understand how the electron configuration of nitrogen, with its atomic number of 7, is determined.
Nitrogen has seven electrons, which occupy the atomic orbitals in a specific order. The first two electrons fill the 1s orbital, while the next two electrons go into the 2s orbital. So far, nitrogen has four electrons, leaving three more to be placed.
Now, according to the Pauli Exclusion Principle, the remaining three electrons must occupy three different orbitals with different spins. This means that two electrons fill the 2p orbital with opposite spins, and the final electron occupies a separate 2p orbital with a different spin.
Overall, nitrogen’s electron configuration is 1s2 2s2 2p3. This configuration implies that nitrogen has two electrons in the first energy level (1s orbital), two electrons in the second energy level (2s orbital), and three electrons in the second energy level (2p orbitals).
This unique electron configuration gives nitrogen its chemical properties and contributes to its ability to form strong covalent bonds with other elements. Understanding the Pauli Exclusion Principle and its implications for nitrogen’s electron configuration is crucial in comprehending the behavior and characteristics of this important element.
In summary, the Pauli Exclusion Principle states that no two electrons in an atom can have the same quantum state. This principle has significant implications for nitrogen’s electron configuration, resulting in its distinctive arrangement of electrons in different orbitals. By adhering to the Pauli Exclusion Principle, nitrogen exhibits unique chemical properties that make it an essential element in various processes and compounds.
Hund’s Rule
Hund’s Rule governs nitrogen’s electron configuration. Each orbital is singly filled, with parallel spins, before pairing occurs. This results in a half-filled p sublevel, as nitrogen follows the 1s2 2s2 2p3 configuration, adhering to Hund’s Rule.
What Is Hund’s Rule?
Hund’s Rule is a fundamental principle in quantum mechanics that governs how electrons occupy atomic orbitals. It states that electrons will first fill empty orbitals of the same energy (degenerate orbitals) with the same spin before pairing up with an opposite spin.
Applying Hund’s Rule To Nitrogen
Nitrogen, with the atomic number 7, has an electron configuration of 1s2 2s2 2px1 2py1 2pz1. To understand how Hund’s Rule is applied, let’s break it down step by step:
1. The nitrogen atom starts with two electrons in the 1s orbital, each with opposite spins.
2. The next two electrons go into the 2s orbital, again with opposite spins.
3. According to Hund’s Rule, the three remaining electrons must occupy the three 2p orbitals (2px, 2py, 2pz). One electron is placed into each orbital, each with parallel spins.
4. By distributing the electrons this way, nitrogen achieves the lowest possible energy configuration, as the Pauli exclusion principle requires.
This arrangement of electrons following Hund’s Rule allows nitrogen to exhibit its unique chemical properties, such as its ability to readily form covalent bonds and participate in chemical reactions.
- Nitrogen’s electron configuration: 1s2 2s2 2px1 2py1 2pz1
- Electrons in 2p orbitals: 2px1, 2py1, 2pz1
Overall, Hund’s Rule plays a crucial role in determining the electron configuration of nitrogen and other elements, guiding the behavior and properties of atoms in the vast realm of chemistry.
The Noble Gas Configuration
Understanding the noble gas configuration is crucial in comprehending the electron configuration of nitrogen. The noble gas configuration refers to the arrangement of electrons in an atom, with reference to the nearest noble gas element. This concept simplifies the notation of electron configurations, making it more concise and easier to understand.
Introduction To Noble Gas Configuration
The noble gas configuration involves using the electron configuration of the nearest noble gas element as a starting point and then adding the remaining electrons for the specific element. This approach provides a shorthand method for representing the electron configuration, particularly for elements with higher atomic numbers.
Using Noble Gas Configuration For Nitrogen
For nitrogen, the noble gas configuration can be utilized by considering the electron configuration of the nearest noble gas, which is helium (He). Helium has an electron configuration of 1s2, and nitrogen has an electron configuration of 1s2 2s2 2p3. By incorporating the electron configuration of helium as the preceding noble gas, we can express the electron configuration of nitrogen as [He] 2s2 2p3.
The Importance Of Nitrogen Electron Configuration
Nitrogen electron configuration is crucial as it determines the element’s chemical properties and reactivity. Understanding its arrangement of electrons can provide insights into bonding and reactions in various compounds and biological processes.
Nitrogen, with its atomic number of 7, is a vital element that plays a crucial role in various aspects of chemistry. One of the key characteristics that contribute to its significance is its electron configuration. Understanding nitrogen’s electron configuration is essential for comprehending its role in organic chemistry, as well as its reactivity and bonding patterns.
Nitrogen’s Role In Organic Chemistry
In organic chemistry, nitrogen is often found in amino compounds, which are the building blocks of proteins and nucleic acids. Nitrogen’s electron configuration, which consists of two electrons in the 1s orbital and five electrons in the 2p orbital, allows it to readily form covalent bonds.
This ability is especially important for organic compounds where nitrogen can form multiple bonds, enabling it to participate in diverse reactions. Nitrogen’s electron configuration enables it to act as both a nucleophile and a Lewis base, making it an indispensable component of various biological substances.
Reactivity And Bonding Patterns
Nitrogen’s electron configuration significantly influences its reactivity and bonding patterns. With its lone pair of electrons in the 2p orbital, nitrogen readily forms bonds with other atoms, particularly those with vacant orbitals.
Because of its strong electronegativity, nitrogen attracts electrons towards itself, making it highly reactive. This characteristic makes nitrogen compounds versatile and allows them to participate in a wide range of chemical reactions, such as the formation of stable covalent bonds and the creation of coordination compounds.
The presence of lone pairs in nitrogen’s electron configuration also makes it an ideal candidate for hydrogen bonding. This property is extensively utilized in biological systems, such as in DNA and protein structures, influencing their stability and function.
By understanding the importance of nitrogen electron configuration, scientists and chemists can gain valuable insights into its behavior and properties, allowing them to study and harness its vast potential in various fields of chemistry.
Nitrogen Electron Configuration Variations
The nitrogen atom is a vital component of many compounds and is crucial in various biological processes. Understanding the electron configuration of nitrogen is essential in comprehending its chemical behavior. But did you know that the electron configuration of nitrogen can vary in different scenarios? In this section, we will explore two key variations: ions and isotopes, and their impact on nitrogen’s electron configuration.
Ions And Nitrogen Electron Configuration
When nitrogen gains or loses electrons, it becomes an ion. Ions can be positively charged (cations) or negatively charged (anions), depending on whether they lose or gain electrons, respectively. The electron configuration of nitrogen ions differs from that of neutral nitrogen due to the gain or loss of electrons.
In the case of a nitrogen cation, one or more electrons are removed from the atom. As a result, its electron configuration is altered. For example, the electron configuration of the nitrogen ion N+ can be represented as 1s2 2s2 2p2. Here, the 2 electrons from the 2p orbital have been removed.
On the other hand, when a nitrogen atom gains electrons to form an anion, its electron configuration undergoes a change as well. Taking the nitride ion (N-) as an example, its electron configuration can be written as 1s2 2s2 2p6. In this case, the 2p orbital is fully occupied with 6 electrons instead of the usual 5 in neutral nitrogen.
Isotopes And Their Impact
Isotopes are atoms of the same element with different numbers of neutrons. Nitrogen has two stable isotopes, Nitrogen-14 and Nitrogen-15. While both exhibit the same electron configuration in their neutral state, their slight difference in mass affects their behavior.
- Nitrogen-14: Contains 7 protons, 7 neutrons, and 7 electrons. Its electron configuration is 1s2 2s2 2p3.
- Nitrogen-15: Contains 7 protons, 8 neutrons, and 7 electrons. Its electron configuration is the same as Nitrogen-14: 1s2 2s2 2p3.
Although the electron configuration remains unchanged, the difference in the number of neutrons can lead to variations in stability and nuclear properties. These isotopic variations are utilized in scientific research and various applications, such as isotopic labeling in biological studies.
In conclusion, understanding the variations in nitrogen’s electron configuration is essential for comprehending its chemical behavior in different contexts. Whether it’s through the formation of ions or the presence of isotopes, these variations play a significant role in the diverse roles nitrogen plays in our world.
Notable Nitrogen Electron Configurations
The electron configuration of nitrogen is of great interest in the study of chemistry due to its unique properties and behavior. Understanding the electron configurations of nitrogen in its ground state and excited state is essential in comprehending its chemical reactivity and bonding capabilities. Let’s delve into the notable nitrogen electron configurations to gain a deeper insight into this vital element.
Nitrogen Electron Configuration In Ground State
In its ground state, nitrogen’s electron configuration is denoted as 1s2 2s2 2p3. This arrangement signifies that in the first shell, there are 2 electrons in the 1s subshell, and in the second shell, there are 2 electrons in the 2s subshell and 3 electrons in the 2p subshell. This configuration provides valuable information about the distribution of nitrogen’s electrons, strongly influencing its chemical behavior and reactivity.
Excited State Electron Configurations
When nitrogen transitions to an excited state, its electron configurations alter, resulting in various possible arrangements. These excited state configurations, denoted as 1s2 2s1 2p4, 1s2 2s2 2p2, and 1s2 2s1 2p5, among others, significantly impact nitrogen’s reactivity and chemical properties. Google maps
Frequently Asked Questions For Nitrogen Electron Configuration
How Do You Write The Electron Configuration For Nitrogen?
Nitrogen’s electron configuration is 1s2 2s2 2p3. It contains two electrons in the 1s orbital, two in the 2s orbital, and three in the 2p orbital.
What Element Is 1s2 2s2 2p6 3s2 3p6 4s2?
The given electron configuration belongs to the element calcium (Ca). It has 20 electrons and is found in the fourth period of the periodic table.
What Is The Electron Configuration Of Nitrogen 14 7?
The electron configuration for nitrogen-14 (7 protons and 7 electrons) is 1s^2 2s^2 2p^3.
Which Element Does The Electron Configuration 1s22s22p2 Represent?
The electron configuration 1s22s22p2 represents the element oxygen.
Conclusion
The electron configuration of nitrogen is 1s² 2s² 2p³. Understanding this configuration is crucial in chemistry and beyond. The arrangement of electrons in the nitrogen atom impacts its chemical properties and behavior. Exploring nitrogen’s electron configuration provides valuable insight into its role in various processes and industries.