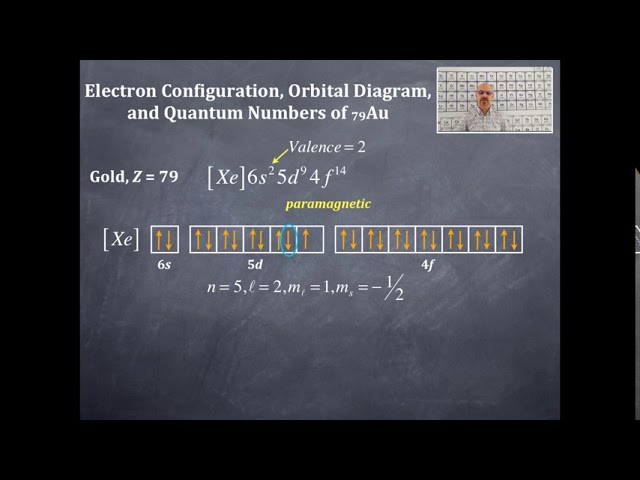
The electron configuration of gold is [Xe] 4f14 5d10 6s1. Gold has 79 electrons distributed among its orbitals, with the 79th electron occupying the 6s orbital.
This unique electronic configuration is responsible for the chemical and physical properties of gold, including its high malleability, ductility, and resistance to oxidation. Gold, with the symbol Au, is one of the most highly coveted metals since ancient times. It is a transition metal and is located in group 11 of the periodic table.
With an atomic number of 79, the electron configuration of gold is [Xe] 4f14 5d10 6s1. It belongs to the d-block of elements and has a unique electronic configuration that makes it highly valuable in various applications, such as jewelry making, investment, and electronics. Gold is also known for its exceptional chemical stability and resistance to corrosion, making it a highly popular material for long-term storage and preservation.
The Basics Of Electron Configuration
Gold’s electron configuration is [Xe] 4f14 5d10 6s1, meaning it has 79 electrons in total. In its ground state, the one unpaired electron is in the 6s orbital, giving it its characteristic yellow color and excellent ductility.
Electron configuration describes how electrons are arranged around the nucleus of an atom. It describes the arrangement of electrons in multiple energy levels or shells. The arrangement of electrons is crucial as it determines an element’s chemical properties. In this section, we will discuss the electron configuration, why it is important, how it is written, and the relationship between the periodic table and electron configuration.
What Is Electron Configuration?
Electron configuration is a representation that shows the number of electrons present in an atom’s electron shell. The electron shells are represented by the letters K, L, M, N, etc., which correspond to the energy level in which the electrons are present. The K-shell corresponds to the lowest energy level, followed by the L-shell, and so on. The number of electrons present in each shell is represented by a superscript number.
Why Is It Important?
Electron configuration is crucial in determining the chemical properties of an element. The electron configuration of an element determines how it interacts with other elements and how it reacts chemically. The properties of an element are determined by the number of valence electrons present in the outermost shell of the atom. The valence electrons are the ones involved in chemical reactions.
How Is Electron Configuration Written?
Electron configuration is written by listing the subshells in order of increasing energy. The subshells are represented by letters: s, p, d, and f. The number of electrons in each subshell is represented by a superscript. For example, the electron configuration of carbon is 1s^2 2s^2 2p^2. This means that carbon has two electrons in the 1s subshell, two electrons in the 2s subshell, and two electrons in the 2p subshell.
The Relationship Between Periodic Table And Electron Configuration
The periodic table is a tabular arrangement of elements based on their chemical and physical properties. The table is arranged in periods and groups. The period indicates the number of energy levels or shells present in the element, while the group indicates the number of valence electrons present. The electron configuration of an element can be determined by its position on the periodic table. In conclusion, electron configuration is the way electrons are arranged around the nucleus of an atom, and it determines the chemical properties of an element. The electron configuration is written by listing the subshells in order of increasing energy. The valence electrons in the outermost shell are responsible for the chemical properties of the element. The periodic table arrangement can help determine the electron configuration of an element, making it a crucial tool for chemists and scientists.
Gold – Characteristics And Properties
Gold’s electron configuration contributes to its unique properties, including its high malleability and conductivity. With a full d sublevel and partially filled s sublevel, gold is considered a transition metal.
Gold is a chemical element with the symbol Au and atomic number 79. This precious metal is highly sought after for its bright yellow color and noble properties. Its atomic structure allows it to have unique characteristics and properties, making it a valuable component in various industries. In this section, we will discuss the atomic structure of Gold, its Physical Properties, its Chemical Properties, and its Uses in detail.
Atomic Structure Of Gold
Gold belongs to the gold group and has a configuration of [Xe] 4f^14 5d^10 6s^1. It has a nucleus consisting of 79 protons and 118 neutrons, with electrons orbiting the nucleus. The valence electron of Gold resides in the 6s subshell, making it highly reactive in chemical reactions. The atomic radius of Gold is 174 pm, and it has a density of 19.3 g/cm^3. Its melting point is 1064°C, and the boiling point is 2856°C.
Physical Properties Of Gold
Gold metal has a distinctive bright yellow color and a shiny texture that makes it highly attractive to use for jewelry. This metal is highly ductile and malleable, which means it can be drawn into thin wires or flattened into sheets easily. Gold is a good conductor of electricity and does not tarnish or corrode under normal conditions. Moreover, Gold is highly resistant to acids and bases, making it useful in various chemical applications.
Chemical Properties Of Gold
Gold is a noble metal, which means it is relatively unreactive compared to other metals. It is resistant to oxidation, and its (+1) oxidation state is the most common. Gold is a good reducing agent, and it can reduce metal ions to their respective metal. When Gold combines with other elements, it forms various compounds such as Gold chloride, Gold cyanide, and Gold oxide compounds.
Uses Of Gold
Gold has various uses in industry, medicine, and technology. The most prominent use of Gold is in jewelry and coinage. It is also used in dentistry, electronics, and aerospace industries. Gold nanoparticles are used in cancer treatment and diagnostics. The unique properties of Gold make it suitable for use in various applications such as mirrors, space helmets, and coatings. In conclusion, Gold is a unique precious metal with distinct characteristics and properties. The Atomic Structure of Gold, Physical Properties of Gold, Chemical Properties of Gold, and Uses of Gold have been explored in detail, highlighting the importance and versatility of this metal.
The Electron Configuration Of Gold
As an element, gold has fascinated humans for centuries. It is a precious metal that has been used for everything from making jewelry and coins to dental fillings and electronics. But what is the electron configuration of gold, and how does it contribute to the properties of this element? In this section, we will take a closer look at the electron configuration of gold, both in its ground state and when it is in an excited state. We will also discuss how to write the electron configuration of gold using the appropriate notation.
Overview Of Gold Electron Configuration
The electron configuration of an element describes the distribution of electrons in its atomic orbitals. It is an important factor in determining the element’s chemical and physical properties, such as its reactivity, melting point, and conductivity. Gold has an atomic number of 79, which means it has 79 protons and 79 electrons. In its ground state, the electrons in gold are arranged in four energy levels, or shells, around the nucleus.
Gold Electron Configuration In Ground State
The gold electron configuration in its ground state can be written as 1s22s22p63s23p64s23d104p65s24d105p66s1. This notation indicates that there are two electrons in the first shell, eight electrons in the second shell, 18 electrons in the third shell, 32 electrons in the fourth shell, and one electron in the fifth shell. The outermost electron in gold is in the sixth shell and has an energetic level of 6s1.
Gold Electron Configuration In Excited State
When gold is in an excited state, one or more of its electrons are temporarily promoted to a higher energy level. The gold electron configuration in an excited state can be written as 1s22s22p63s23p64s23d104p65s24d105p65d106s1. This notation shows that one electron from the fifth shell has been promoted to the fifth d orbital. The electron in the d orbital has higher energy than the electron in the 6s1 orbital.
How To Write Gold Electron Configuration
The electron configuration of gold can be written using either the shorthand notation or the full orbital notation. The shorthand notation for the ground state configuration of gold is [Xe] 4f145d106s1. The [Xe] represents the electron configuration of xenon since the electron configuration of gold can be viewed as the xenon configuration plus one electron in the sixth shell. In the full orbital notation, the electron configuration is written out, as we have done above. This notation shows the distribution of the electrons among the different orbitals.
Relationships Between Gold Electron Configuration And Characteristics
The gold electron configuration plays a significant role in determining its physical and chemical characteristics. With 79 electrons, gold has a unique electron configuration, which leads to its exceptional ductility, electrical conductivity, and corrosion resistance.
How Gold Electron Configuration Determines Chemical Properties
226261016110. This configuration determines the chemical properties and behavior of gold. The valence electrons, which are the outermost electrons, determine reactivity and bonding tendencies. Gold has only one valence electron, which makes it chemically stable and resistant to oxidation or corrosion. This stability makes it an excellent conductor of electricity and a popular choice in industrial and electronic applications.
Differences In Properties Between Gold And Other Elements
Due to its unique electron configuration, gold has many distinct properties that differentiate it from other elements in the periodic table. For example, it has a higher melting point and boiling point than most other metals due to the strength of its metallic bonds. Additionally, gold does not react with most other elements, making it non-toxic and biologically inert, making it a safe material for medical implants.
Relationship Between Gold Electron Configuration And Color
Gold’s electron configuration also plays a role in the striking color of the metal. Gold appears yellow because of its unique electronic structure. The d-band electrons in gold absorb light in the blue-green region of the spectrum, giving the gold a yellow appearance. The purity of gold also affects its color, with higher-purity gold appearing brighter and more yellow. In conclusion, gold’s electron configuration determines its chemical properties, behavior, and unique characteristics that differentiate it from other elements. The properties of gold are useful in many industrial and electronic applications, and it remains a popular choice due to its non-toxicity, resistance to corrosion, and striking color.
Current Research And Applications Of Gold Electron Configuration
The electron configuration of gold plays a crucial role in various fields of research and technology. Gold’s distinctive nature and stability make it an essential element; thus, further research on its electron configuration is pertinent to better comprehend its scientific and technological utilities. In this blog post, we will explore the current research on the gold electron configuration and its application in technology.
Research In Gold Electron Configuration
Various researchers and scientists are studying the electron configuration of gold and its implications. They are exploring new methods and techniques to understand better and utilize the gold configuration to make advancements in different industries.
One such research is on the “plasmon excitation” of gold nanoparticles. Plasmon excitation refers to the concentration of an electron density in response to light excitation. The research showed that gold nanoparticles generate a plasmon resonance effect, which could be useful in creating new sensors or improving current medical imaging technologies.
Another research involves the electron configuration effect in the synthesis of gold nanoclusters. Scientists have discovered that changing the arrangement of gold atoms in nanoclusters changes their properties and opens up new avenues for potential applications in industries like electronics, biomedicine, and catalysis.
Applications Of Gold Electron Configuration In Technology
The research on gold electron configuration has led to several practical applications in technology. The following are some of the notable applications:
|
TABLE: Notable Applications of Gold Electron Configuration in Technology |
|
|
Applications |
Description |
|---|---|
|
Catalysis |
Gold nanoparticles with specific electron configurations act as catalysts, making reactions faster and more efficient. This is essential in industry applications like energy conversion and chemical synthesis. |
|
Medical Imaging |
Gold nanoparticle electron configuration can enhance the contrast in medical imaging, such as computed tomography (CT) scans, resulting in more accurate diagnosis and treatment of diseases. |
|
Electrical Conductivity |
Gold is an excellent conductor of electricity, and its unique electron configuration enhances conductivity making it a valuable element in electronics and electronic devices production. |
In conclusion, studying the gold electron configuration has led to new innovations in different fields of research and technology. Its unique electronic structure and stability make it an essential element with practical applications in several industries. Further research in this domain will undoubtedly lead to more discoveries and advancements.
Gold Electron Configuration Misconceptions And Controversies
Gold’s electron configuration has long been a topic of debate and controversy due to common misconceptions. Despite being a transition metal, some argue that gold’s configuration should follow the rules of the s block. However, current scientific consensus agrees that gold’s electron configuration accurately reflects its position in the d block.
Gold has been a precious metal for ages, with a fascinating history that boasts of its use in various fields. Gold electron configuration, which is the arrangement of gold’s electrons, is often discussed among scientists, researchers, and enthusiasts. However, there are numerous misconceptions and controversies surrounding the configuration of gold electrons. In this section, we will explore these myths and controversies that have puzzled the scientific community for years.
Misconceptions About Gold Electron Configuration
There are a few misconceptions surrounding gold electron configurations that have misled individuals into believing certain things that are far from the truth. One common myth is that gold has its maximum capacity with only six valence electrons, which is inaccurate. In reality, gold prefers to have one or three valence electrons to obtain electron configurations similar to copper and silver. Another misconception is that gold is monovalent, meaning it can only form one type of ion. This is false, as gold can form several ions, including Au+, Au2+, and Au3+. The misconception is likely due to the fact that the Au+ ion is the most stable. It is also a common belief that gold has a full valence shell, which is incorrect since gold has only one incomplete valence shell.
Controversies Surrounding Gold Electron Configuration Experiments
The configuration of gold electrons is a topic of great interest to scientists, and numerous experiments have been conducted to determine the correct configuration. However, some of these experiments have raised questions due to their controversial results. One such experiment is the photoelectron spectroscopy experiment, which contradicted the expected results by displaying a valence shell electron configuration of 5d106s1 instead of the predicted 5d96s2 configuration. Another disputed configuration experiment is the extended X-ray absorption fine structure spectroscopy, which showed an icosahedral arrangement of gold atoms in a gold-thiolate cluster. This study’s results were controversial because it led to debates about the actual configuration of gold atoms. Despite the controversies surrounding these experiments, they have played a significant role in shaping our understanding of gold electron configurations. In conclusion, gold electron configuration is a topic of great interest and importance in the scientific community. Although numerous experiments have been conducted to determine its configuration, controversies and misconceptions still surround the topic. By understanding and addressing these controversies and misconceptions, we can enhance our knowledge of gold and its electron configurations.
Frequently Asked Questions Of Gold Electron Configuration
How Do You Write The Electron Configuration For Gold?
The electron configuration for gold is [Xe] 4f14 5d10 6s1.
What Is The Electron Configuration Of Gold Au 79?
The electron configuration of gold (AU 79) is [Xe] 4f14 5d10 6s1.
What Is The Electronic Configuration Of Golden?
The electronic configuration of gold is [Xe] 4f14 5d10 6s1.
How Do You Write The Electronic Configuration Of Au?
To write the electronic configuration of Au, we can follow Aufbau’s principle using the noble gas notation. The atomic number of Au is 79, and its electronic configuration is 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶.
Conclusion
The gold electron configuration has been a topic of interest for decades due to its unique properties and numerous applications. Through this blog post, we have delved into the intricacies of electron configurations, discussed the history of gold, and explored its various uses.
Whether you are a scientist, student, or simply interested in the subject matter, this information will undoubtedly help you better understand gold’s electronic structure and what makes it such a valuable element. So, go ahead and share your newfound knowledge with those around you, and keep learning!