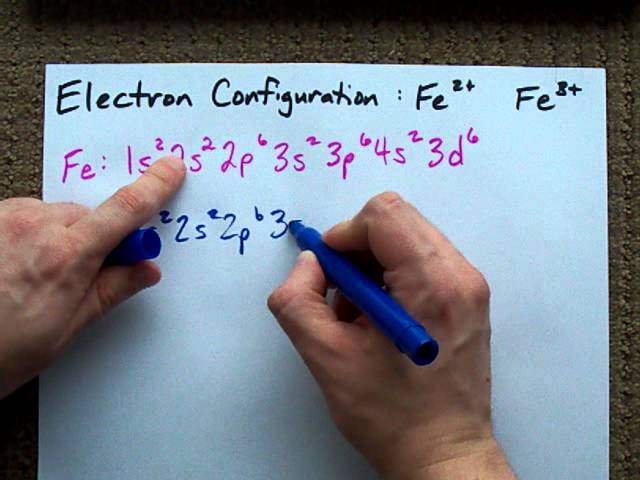
The electron configuration of Fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6. Fe2+ has lost two electrons from its outermost shell, leaving behind a filled d-subshell and an empty s-subshell.
Iron (Fe) is a transitional metallic element that can form various ions, including Fe2+. The 3rd energy level contains 8 electrons in the s and p orbitals. The electron configuration of Fe is 1s2 2s2 2p6 3s2 3p6 3d6 4s2.
However, when it loses two electrons to form Fe2+, the electron configuration becomes 1s2 2s2 2p6 3s2 3p6 3d6. The Fe2+ ion has a +2 charge, meaning it has two fewer electrons than neutral iron. This loss of electrons eliminates the 4s electrons, leaving the ion with a partially filled 3d sublevel. Understanding the electron configuration of Fe2+ is crucial in predicting its chemical and physical properties in various applications.
Frequently Asked Questions For Electron Configuration Of Fe2+
What Is The Electronic Configuration Of Fe2+ Unpaired Electrons?
The electronic configuration of Fe2+ has four unpaired electrons, thus giving it a magnetic moment.
What Is The Electron Configuration Of Iron Fe?
The electron configuration of iron (Fe) is 1s2 2s2 2p6 3s2 3p6 4s2 3d6.
How Many Electrons Are In Fe2+ Ion?
Fe2+ ion contains two electrons.
What Is The Electron Configuration Of Ni2+?
The electron configuration of Ni2+ is [Ar] 3d8.
Conclusion
Understanding the electron configuration of Fe2+ allows us to comprehend its chemical and physical properties. It also helps us predict its behavior in various reactions and environments. Knowing the electron configuration enables us to understand how Fe2+ interacts with other elements.
Hence, the electron configuration of Fe2+ is an essential concept in chemistry, and it is critical to grasp its significance.