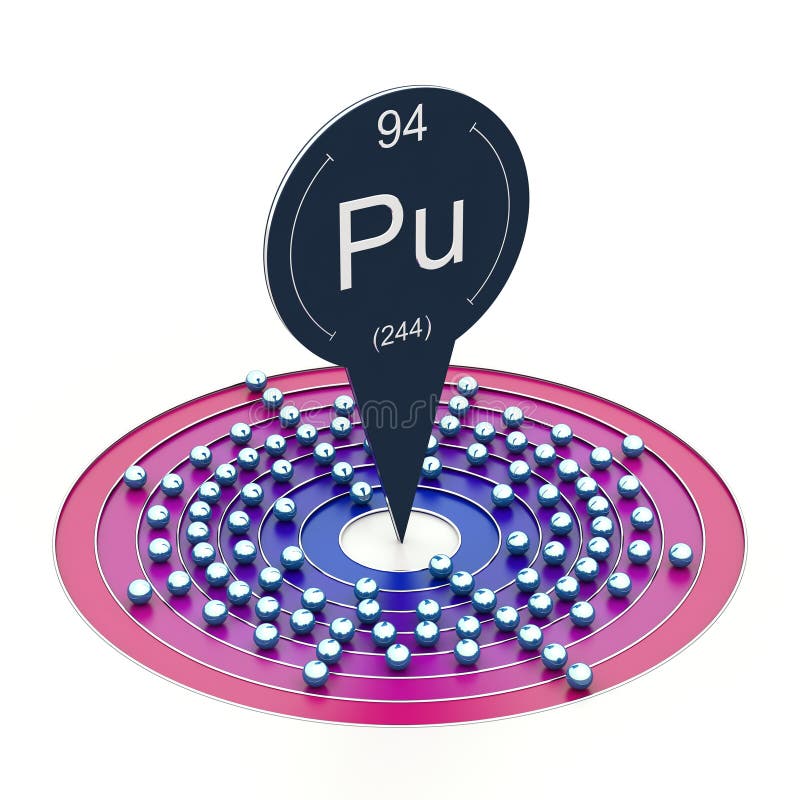
Plutonium electron configuration is [Rn] 5f6 7s2. It is a radioactive element with atomic numbers 94 and 94 electrons distributed across different electron shells.
Plutonium’s electron configuration indicates the arrangement of these electrons in various energy levels around the nucleus. This information helps determine the element’s chemical behavior and reactivity with other elements. Plutonium is commonly used in nuclear reactors and weapons due to its ability to undergo nuclear fission, which releases substantial energy.
Despite its usefulness, Plutonium is also hazardous to health due to its radioactivity. Understanding its electron configuration is vital in handling and disposing of it safely.
The Electron Configuration Of Plutonium
Plutonium’s electron configuration is [Rn] 5f6 7s2, which means there are 94 electrons in total. Due to the placement of its electrons in the 5f shell, Plutonium has a very complex electron configuration.
Overview Of Electron Configuration
The electron configuration of an atom is the distribution of its electrons into different energy levels and sub-levels. The arrangement of electrons in an atom is determined by the Aufbau principle, which states that electrons fill orbitals in order to increase energy. Plutonium, with an atomic number of 94, has a complex electron configuration due to the presence of many electrons.
Ground State Electron Configuration
The ground state electron configuration of plutonium can be written as [Rn] 5f6 7s2. This notation indicates that the 94 electrons in the plutonium atom are arranged such that the first 86 electrons occupy the energy levels of the noble gas radon, denoted by the symbol Rn. The remaining 8 electrons occupy the sub-levels of the 5f and 7s energy levels.
Excited State Electron Configuration
When an atom is excited by absorbing energy in the form of light or heat, one or more electrons can move to a higher energy level or sub-level. The excited state electron configuration of plutonium can be represented as [Rn] 5f5 6d1 7s2. In this configuration, one electron is promoted from the 5f sub-level to the higher energy 6d sub-level.
Explanation Of Electron Configuration Notation
The electron configuration notation demonstrates the distribution of electrons amongst the orbitals of an atom. In this notation, the principal quantum numbers are represented by the letters K, L, M, N, O, P, and Q, with K being the closest to the nucleus. Each letter represents an energy level, and the subscript denotes the number of electrons at that energy level. The letter(s) and number(s) in brackets represent the electron configuration of the noble gas that is closest in atomic number to the atom of interest. Overall, understanding the electron configuration of plutonium is crucial in understanding its chemical properties and behavior in various chemical reactions.
Factors Affecting Electron Configuration
Plutonium’s electron configuration is affected by its atomic number and the energy levels of its electrons. Other factors, such as the shielding effect and the nuclear charge, also impact electron configuration. Understanding these factors is crucial for studying the properties and behavior of plutonium and other elements.
Factors Affecting Electron Configuration: Electron configuration is essential in understanding the behavior of atoms in reactions. The electron configuration of an atom refers to its placement of electrons in shells and sub-shells of the atom. Each element has a unique electron configuration, and several factors affect electron configuration. Understanding these factors, like the effect of atomic number, nuclear charge, and shielding, helps understand how the valence electrons are affected and why atoms react differently. Effect of Atomic Number: The number of electrons in an atom is highly dependent on its atomic number. The atomic number is the unique identifier of an element and indicates the number of protons in its nucleus. The number of protons affects the number of electrons and, thus, the electron configuration. As the atomic number increases, the number of electrons also increases, resulting in different electron configurations amongst elements. Effect of Nuclear Charge: The nuclear charge is the number of protons in an atom’s nucleus, attracting the electrons towards the nucleus. When the nuclear charge increases, the electrons in the atom feel a stronger attraction toward the nucleus, leading to a tight grip on the valence electrons. This tighter grip results in a more compact and stable electron configuration, making removing electrons from the atom harder. Effect of Shielding: The shielding effect refers to the reduction in the nuclear charge that valence electrons feel due to electron shielding. When electrons occupy inner shells, they shield or block the nuclear charge from the valence electrons in the outermost shell. This screening of the valence electrons affects the electron configuration since the valence electrons only experience a fraction of the entire nuclear charge and, therefore, result in a different configuration. For instance, lower levels of shielding create a stronger attraction between the valence electrons and the nucleus, leading to a compact electron configuration. In conclusion, many factors affect electron configuration, such as atomic number, nuclear charge, and shielding. Although they may seem disconnected, gaining knowledge of these factors can help you understand the trends in the periodic table, chemical reactivity, and the physical properties of elements.
Applications Of Plutonium Electron Configuration
Plutonium electron configuration has various practical applications in nuclear reactors, nuclear weapons, and other nuclear technologies. Understanding plutonium’s electron configuration can help scientists predict its chemical and physical properties, such as its reactivity and stability, which are essential for designing safe and efficient nuclear systems.
Plutonium, a radioactive element, has a complex electron configuration that makes it useful in various fields. Here are some applications of plutonium electron configuration.
Radioactive Decay
Plutonium undergoes spontaneous fission, producing large amounts of heat and radiation. This property makes it useful in nuclear batteries and spacecraft power sources. Plutonium-238, an isotope of plutonium, is particularly useful in powering deep-space probes due to its long half-life.
Nuclear Reactors
Plutonium is an essential element in nuclear reactors, as it can undergo fission and release energy in the form of heat. This heat is harnessed to produce electricity, making nuclear power a reliable and cost-effective energy source. Plutonium is also used as a fuel in fast-breeder reactors, which can produce more fuel than they consume.
Nuclear Weapons
Plutonium is a key component in the production of nuclear weapons. Its ability to undergo fission and release immense amounts of energy makes it a powerful tool for destructive purposes. However, the use of plutonium in nuclear weapons is highly regulated and restricted to ensure its safe and responsible use. In conclusion, the electron configuration of plutonium has various applications in different fields. From powering space probes to producing energy in nuclear reactors and even in the creation of nuclear weapons, plutonium’s unique properties make it a valuable element.
Environmental And Health Concerns
Plutonium is a radioactive element that has both environmental and health concerns. Due to its use in nuclear weapons and power generation, plutonium is a heavily regulated substance.
Sources Of Plutonium
Plutonium is commonly found in the environment, particularly in soil. However, the majority of plutonium is man-made and a byproduct of nuclear reactions.
| Source of Plutonium | Description |
| Nuclear weapons testing | The use of plutonium in nuclear weapons tests has led to contamination of soil and water sources. |
| Nuclear power plants | Spent nuclear fuel from power plants contains significant amounts of plutonium, which must be properly stored to prevent contamination. |
| Military nuclear facilities | Plutonium is used in the production of nuclear weapons and can be released into the environment through accidents or incidents. |
Toxicity And Radioactivity
Plutonium is a highly radioactive toxic substance that can cause serious health problems when ingested, inhaled, or absorbed by the skin. The alpha radiation released by plutonium can cause significant cellular damage, leading to cancer and other health issues.
Due to its radioactivity, plutonium must be handled with extreme caution to prevent exposure. In addition to the health risks posed by plutonium, it is also a significant environmental hazard, as it can persist in the environment for thousands of years.
Safety Measures And Regulations
Due to the significant risks associated with plutonium, various safety measures and regulations have been implemented to protect workers and the general public from exposure. These measures include:
- Use of protective clothing and equipment when handling plutonium
- Proper storage and disposal of plutonium waste
- Regular monitoring of workers and the environment for potential contamination
- Regulation of the production, use, and transport of plutonium
Overall, safety measures and regulations are essential to minimize the environmental and health risks associated with plutonium.
Future Of Plutonium Research
Plutonium is a radioactive and highly toxic chemical element. Its properties make it ideal for use in nuclear reactors, nuclear weapons, and space missions. Plutonium is also a potential energy source, which has led many researchers to study it in more detail. In this post, we’ll explore the current research on plutonium, its potential applications, and future challenges and opportunities.
Current Research On Plutonium
The properties of plutonium make it one of the most complex elements for scientists to study. Currently, most researchers are focused on understanding its physical and chemical properties. Some of the areas of research include:
- Investigating plutonium’s reactivity with other elements
- Studying its behavior in various environments, such as water or air
- Developing more efficient methods of producing plutonium
Potential Applications
Plutonium has many potential applications that can benefit humanity. Some of the most common include:
| Application | Description |
|---|---|
| Nuclear Power | Plutonium can be used as a fuel for nuclear reactors, a renewable energy source. |
| Nuclear Weapons | Plutonium is a key component of nuclear weapons, which helps maintain national security. |
| Space Exploration | Plutonium is used to power spacecraft on long-duration space missions, where solar power is not feasible. |
Future Challenges And Opportunities
Despite its potential applications, plutonium presents many challenges to researchers. One of the biggest challenges is its safe handling and storage. Plutonium is highly toxic and can risk human health and the environment if mishandled.
Another challenge is developing more efficient methods of producing plutonium. The current method of producing plutonium is through nuclear reactors, which is a slow and expensive process. Researchers are exploring new methods, such as particle accelerators, to produce plutonium more efficiently.
Despite these challenges, the future of plutonium research looks promising. As new technologies emerge and our understanding of this element expands, we expect many new applications and discoveries in the coming years. Google maps
Frequently Asked Questions On Plutonium Electron Configuration
What Is The Electron Configuration Of Plutonium?
The electron configuration for plutonium is [Rn]5f6 7s2.
What Element Has An Electron Configuration Of 1s 2 2s 2 2p 6 3s 2 3p 4?
The element with an electron configuration of 1s2 2s2 2p6 3s2 3p4 is sulfur (S).
What Element Has 1s 2 2s 2p 6 3s 2 3p 5 As Its Electron Configuration?
Chlorine (Cl) is the element with the electron configuration of 1s 2 2s 2p 6 3s 2 3p 5.
What Is The Notation For Plutonium?
The notation for plutonium is Pu.
Conclusion
The electron configuration of plutonium is a complex topic that is highly significant in nuclear science. Understanding the electron configuration is crucial for comprehending the element’s properties and applications. With a total of 94 electrons, the configuration of Plutonium is divided into several energy levels.
Through this article, we hope to have provided a comprehensive understanding of the plutonium electron configuration and its role in modern-day technology.