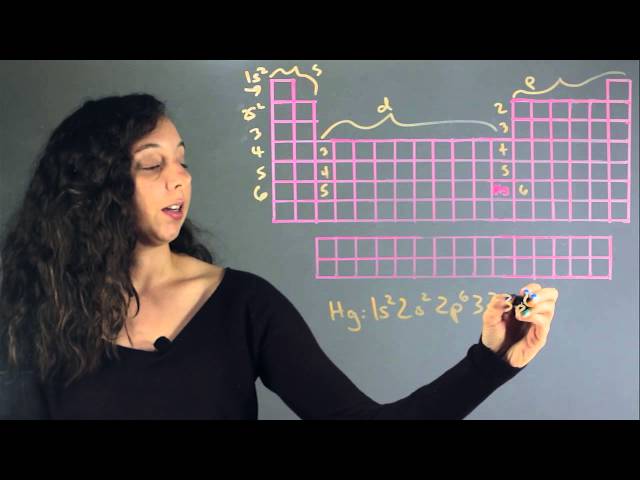Electron Configuration for Mercury is [Xe] 4f14 5d10 6s2. A chemical element with the symbol Hg and atomic number 80, mercury is a transition metal and the only metallic element that is liquid at standard room temperature and pressure.
Mercury has a very high density and is known for its use in thermometers, barometers, and fluorescent lamps. Due to its unique properties, mercury plays an important role in various industries, including the medical and automotive industries. However, its toxicity is a major concern, as it can cause severe health problems if not handled properly.
We will explore the electron configuration of mercury in more detail and understand its significance in chemistry.
Electron Configuration For Mercury
Mercury, with an atomic number of 80, has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2. This means it has two electrons in its innermost shell, followed by two in the second shell, eight in the third, eighteen in the fourth, and two in the fifth.
Mercury is a chemical element with the symbol Hg and atomic number 80. It is a dense, silvery-white liquid at room temperature with a boiling point of 356.73°C. Mercury’s electron configuration is significant as it helps us understand its chemical and physical properties. The configuration refers to the arrangement of electrons in the atom’s shells and subshells.
Ground State Electron Configuration
The ground state electron configuration of mercury is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2. Each number and letter represents a shell and a subshell containing maximum electrons. The first number, 1s2, represents the innermost shell, which can hold only two electrons. The last shell, 6s2, can hold up to 32 electrons.
Valence Electron Configuration
The valence electron configuration of mercury refers to the arrangement of electrons in the outermost shell of the atom. The outermost shell of mercury is the sixth shell, containing two s-electrons and six p-electrons. Thus, the valence electron configuration of mercury is 6s2 6p6. In conclusion, understanding the electron configuration of mercury is essential to comprehend its chemical and physical characteristics, including its unique properties as a liquid metal.
Applications Of Mercury’s Electron Configuration
Mercury’s electron configuration has various applications in lighting, medical, dental, and electrical industries. It is used in fluorescent lights, mercury vapor lamps, thermometers, and dental fillings. Mercury’s unique electronic properties make it useful for various technological applications.
Understanding Chemical Bonding
Mercury is a unique element due to its electron configuration. Its outermost, the valence shell, is incomplete, containing only two electrons. This configuration affects how mercury reacts with other elements and compounds, making it useful in understanding chemical bonding behavior in environmental chemistry.
Behavior In Environmental Chemistry
The electron configuration of mercury makes it highly reactive, especially with halogens such as chlorine and bromine. This reactivity can cause environmental problems, as mercury can combine with other elements to form harmful compounds that can negatively impact plants and animals. However, understanding its behavior in environmental chemistry can also help scientists develop methods to remove these harmful compounds from the environment.
Example: Mercury’s Use In Thermometers
One practical application of mercury’s electron configuration is in thermometers. The unique properties of mercury, such as its high boiling point and low freezing point, make it ideal for measuring temperature. The mercury in a thermometer is in a closed tube, meaning that it doesn’t come into contact with the environment, preventing potential harm to living organisms. By understanding the properties of mercury, scientists were able to create a widely used measuring tool. In conclusion, understanding the electron configuration of mercury can provide insight into its chemical behavior and environmental impacts. Its unique properties have made it useful in various applications, including thermometers. By studying mercury, scientists can better understand how elements and compounds interact, potentially leading to the development of new methods to mitigate environmental harm. Google maps
Frequently Asked Questions For Electron Configuration For Mercury
How Do You Write The Electron Configuration For Mercury?
The electron configuration of mercury is [Xe] 4f14 5d10 6s2.
What Element Has The Electron Configuration 1s2 2s2 2p6 3s2 3p6 4s2?
The element with the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 is Calcium (Ca).
What Is The Electron Configuration Of 1s 2 2s 2 2p 6 3s 2 3p 5?
The electron configuration of 1s2 2s2 2p6 3s2 3p5 is [Ne] 3s2 3p5, where [Ne] represents the electron configuration of Neon (10 electrons).
What Is The Electron Configuration For Hg Z 80 )?
The electron configuration for Hg (Z 80) is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10.
Conclusion
In short, understanding the electron configuration of mercury is crucial for comprehending its chemical properties and reactions. The unique electronic structure of this transition metal plays a vital role in its stability and interactions with other elements. By unraveling the mystery behind this element’s electron arrangement, scientists can develop better methods for controlling and using it in technology and industry.
So, mastering the details of mercury’s orbitals is undoubtedly worth the effort for chemists and physicists alike.